The Flamingo’s Smile (23 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould

Briggs, Clarkson, and Aldridge therefore conclude, with ample justice in my opinion, that the conodont animal is unique and previously unknown. It must be placed in a separate phylum—the Conodonta. After all, they argue, if a century of efforts to squeeze them into some modern group have been dashed on the enigma of their peculiar hard parts, why should the discovery of equally ambiguous soft parts comfortably fit them into some well-established pigeonhole of our taxonomy? They write: “The lack of a definitive solution to this problem in 125 years of research emphasizes the uniqueness of conodonts.” And with this conclusion—that conodonts must be placed in a new and separate phylum of their own—we finally come to the general message that inspired me to write this essay.
Paleontologists are, in general, a conservative lot. Problematica of uncertain taxonomic affinity and few species are an embarrassment and an untidy bother; nothing makes an old-style paleontologist happier than the successful housing of problematical organisms within a well-known group. The admission that Problematica must be treated by erecting new phyla flies in the face of hope and tradition and represents a last resort. In recent years, that resort has been followed more and more often because—well, damn it—many Problematica are weird, wonderful, and unique and simply do not fit into any known group. This most unwilling admission reflects an important and little-known fact about the history of life.
To appreciate this fact and its implications, we must study the distribution in time of Problematica that cannot be placed into conventional phyla. The history of life has featured multicellular animals only during the past 600 million years. We divide this time into three great eras—the Paleozoic (or ancient life), the Mesozoic (or middle life), and the Cenozoic (or recent life). Virtually all Problematica now begrudgingly granted their own phylum lived during the oldest era, the Paleozoic (although conodonts, after living throughout the Paleozoic, just sneaked into the Triassic, the first period of the Mesozoic). This fact, the focal point of my essay, may not strike you, at first, as strange. After all, the further back we go, the more different should life become from our modern phyla. But two aspects of this distribution in time are surprising and point to a major pattern. First, although we might expect a general decrease in the number of problematic groups through time, we would not anticipate an abrupt disappearance of oddities after the Paleozoic. We do not find a gradual decline in curious creatures. Instead, they abound in the lower Paleozoic, become rare by the end of the Paleozoic, and cease thereafter. Of the three windows I mentioned, the Burgess Shale (lower Paleozoic) is chock-full of Problematica, the Mazon Creek (upper Paleozoic) sports two, the Solnhofen lithographic limestone (Mesozoic), none. Something about the earliest history of multicellular life encouraged a flowering of Problematica. Something about its later history (and not much later) dried the well completely.
Second—although the conodonts are an exception to this generality—most Problematica are rare, restricted in time, and represented by only a few species. Phyla are supposed to be big groups—arthropods with their 750,000 species of insects, or chordates with their 20,000 species of fishes. They are also supposed to endure for a long time. Taxonomists are stingy; they do not like to establish a group just below the highest level of kingdom for just a few species that lived but a few million years. If Problematica were restricted to the Paleozoic but were all as abundant and long-lived as conodonts, the pattern would not be as troubling or curious. But some Problematica, now housed in their own phylum, are known as only one species found in a single place. And some are surpassingly strange. Consider the animal so formidably curious that it goes by the Latin name
Hallucigenia
, coined by its author, Simon Conway Morris, for “the bizarre and dream-like appearance of the animal.” (Simon once told me that it resembled something he had seen on a trip—and I don’t mean to Boston.)
Hallucigenia
(from that first and most famous window, the Burgess Shale) has an elongate body, nearly an inch in length, supported by seven pairs of spines that look nothing like the legs of any known creature. It has a bulbous head and, behind it, a row of tentacles, each forked at the tip, running along the back. Behind the tentacles lies a smaller and bunched array of projections recalling the spines on a
Stegosaurus
’s tail. An anal tube projects upward at the rear end (see figure on chapter 16). Damned strangest thing I’ve ever seen in my life. Or consider the peculiar Problematica from the second window, our own Mazon Creek Formation of Illinois. It also bears a whimsical formal name, a Latinization of its discoverer, a Mr. Tully, and its appearance. It is called
Tullimonstrum
. The Tully monster is a peculiar, roughly banana-shaped creature, some three to six inches long. Like
Hallucigenia
, it is so different from anything else we know that it seems to demand a phylum of its own.
We tend to think of evolution as progressive change within lineages—fish become amphibians, reptiles, mammals, and finally humans—and we therefore miss important themes related to a different and more pervasive aspect of evolution: changing diversity, considered as absolute numbers of species and their relative abundance through time. The predominance of Paleozoic Problematica records an outstanding theme in the history of diversity. This theme imparts a direction to time that is more clear and reliable than any statement we can make about change within lineages. It also probably reflects a more general and basic law about the history of change in natural systems.
During the past decade, paleontologists have hotly debated the pattern of change through time in the diversity of marine animals. Do more species live now (as the “progressivist” view of evolution might suggest) or has the number of species remained roughly constant following a quick achievement of some equilibrium value after the Cambrian explosion? The problem is not so easy to solve as it may seem. You can’t simply count the number of species described for each interval of time. The fossil record is notoriously imperfect, and it tends to get worse the further back you go. Thus, an empirical increase in abundance of known fossils could actually reflect a decrease of true diversity.
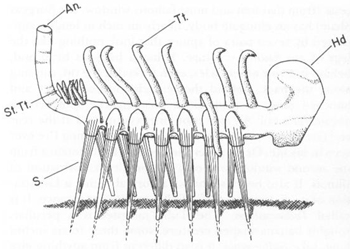
Simon Conway Morris’s restoration of
Hallucigenia sparsa
. Note the seven pairs of spines below (labeled S), the bulbous “head” (in front, labeled Hd), the single row of forked tentacles on the back (labeled Tt), the bunched array of projections at the rear (labeled St. Tt.) and the upstanding “anal tube” (labeled An).
FROM PALAEONTOLOGY, VOLUME
20, 1977, p. 628.
Arguments have therefore raged back and forth, but in 1981 the four leading debaters buried the hatchet and published a joint paper of welcome agreement (J.J. Sepkoski, R.K. Bambach, D.M. Raup, and J.W. Valentine, in bibliography). Several sources of data (all corrected as best we can for imperfection of the record) now point to a clear pattern of real increase through time—not steady and progressive but unmistakable as a general direction. Modern oceans contain at least twice the number of species as our average Paleozoic seas.
We might therefore expect—indeed it seems unavoidable—that modern seas would not only contain more species but also more distinct kinds of creatures, more basically different body plans. Yet it is not so. Today, double the number of species are crammed into far fewer groups of higher taxonomic rank. To be sure, we still find several phyla of distinct body plan and low membership—all the wormlike groups with the funny names that no one but specialists know and love: the kinorhynchs, the gnathostomulids, the priapulids, the chaetognaths, already mentioned as a possible home for conodonts, and several others. But our modern seas are dominated by just a few groups—primarily clams, snails, crabs, fishes, and echinoids—each with far more species than any Paleozoic phylum ever attained (with the possible exception of trilobites in the Ordovician and crinoids in the Carboniferous). Paleozoic seas may have contained only half the species that grace our modern oceans, but these species were distributed over a greatly expanded range of basic body plans. This steady decrease in kinds of organic designs—all in the face of a strong increase in numbers of species—may represent the most outstanding trend of our fossil record.
This steady decrease is well recorded by the pattern of Problematica already discussed. Most of the really weird and wonderful creatures lived exclusively during the Paleozoic. (Don’t be too impressed by the oddity of some modern minor phyla, for many of them did not arise recently but also have records extending back into the Paleozoic.) It is perhaps even better recorded by changes in the number of classes (next lower taxonomic level) within common phyla. Consider just one example, based on a highly conservative counting of classes made by J.J. Sepkoski of the University of Chicago. Modern echinoderms come in four classes, all of respectable to high diversity: sea urchins (the echinoids already cited as a dominant group), starfishes, sea cucumbers, and crinoids. Yet, sixteen additional classes lived and died within the Paleozoic, and sixteen of the total twenty coexisted during the Ordovician period, some 500 million years ago. None of these sixteen classes (with two possible exceptions) ever reached the diversity displayed today by any of the modern survivors.
The Paleozoic world was very different from ours, with few of a kind distributed over a greatly increased range of basic body forms.
Hallucigenia
is gone, the Tully monster lives no longer, even the abundant conodonts are extinct. Why has our world of life undergone this profound shift from few species in many groups to many species in fewer groups?
Of the two general answers, the first is conventional and causal (the second will be based on random processes). It invokes what may be a common property of nearly all natural systems and may therefore have an importance far transcending this particular example. The principle might be called “early experimentation and later standardization.” Some 600 million years ago, the Cambrian explosion filled the oceans with their first suite of multicellular animals. Evolution probed all the limits of possibility. Each basic body plan experimented with a great array of potential variants. The pattern of many groups, each with few members, was established. Some of these experiments worked well, but, inevitably, most didn’t—and a gradual sorting-out ensued.
Many of the failures were flawed from the start and never reached high diversity. They are our taxonomic embarrassments—highly distinct body plans with few species. We call them Problematica and grant them their own phyla only begrudgingly (although if we understood the principle that they represent, we would propose and accept their special names with more equanimity). Others, like the small and extinct classes of Paleozoic echinoderms, are failed experiments with a basic design that does work well in a few successful classes. Thus, sea urchins and starfishes use the echinoderm ground plan to great advantage, while a host of early experiments, bearing such strange names as ctenocystoids, helicoplacoids, and edrioblastoids, quickly bit the dust. Our modern faunas are the winnowed and well-honed survivors of a grand sorting-out based on principles of good engineering.
The same principle applies to any system free to experiment but ultimately regulated by good and workable design. Electric and steam cars, and a variety of other experiments, yielded to the internal combustion engine (although someday, if we ever run out of oil, they may reemerge like the phoenix). Cars now come in hundreds of brands, each built on the same principle. In 1900, far fewer brands used a much greater variety of basic designs. And consider the blimps, gliders, and variety of powered planes before we settled upon 747s and their ilk.
This principle of early experimentation and later standardization dictates a general reduction of variation—particularly the elimination of extremes. We often misunderstand the reason for a loss of extremes because we try to interpret the disappearance of oddities as a trend in its own right and not as an inevitable consequence of decreasing variation within a natural system. Essay 14 on the disappearance of .400 hitters in baseball considers another example of the same process. Conventional explanations for this most striking and widely discussed trend in baseball invariably look for some directional change—introduction of relief pitching or more grueling schedules composed mostly of night games—that would diminish high hitting alone. But I reasoned that the decline of high hitting may simply reflect the stabilization and general perfection of play that must accompany a game as its standards rise (analogous to the reduction of body plans as successful designs predominate in life’s history). As pitching, fielding, and hitting all improve, variation in each category decreases. I was able to show that league averages have not changed between the great era of .400 hitting (1890–1920) and today, but that both highest averages (the .400 hitters)
and
lowest averages have converged toward the league average. In other words, extremes have been eliminated at both ends—the same principle of early experimentation (or toleration) and later standardization.
