What If? (2 page)
Authors: Randall Munroe

. . . and Earth would start turning again.
- 1
I mean, not right away.
- 2
Although without the Coriolis force, it’s anyone’s guess which way they would spin.
- 3
See “Leap Seconds,”
http://what-if.xkcd.com/26
, for an explanation of why this happens.
Relativistic Baseball
Q.
What would happen if you tried to hit a baseball pitched at 90 percent the speed of light?
—Ellen McManis
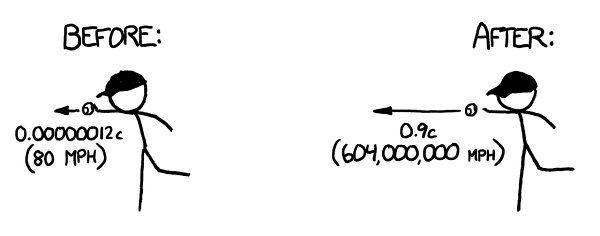
Let’s set aside the question of how we got the baseball moving that fast. We’ll suppose it’s a normal pitch, except in the instant the pitcher releases the ball, it magically accelerates to 0.9c. From that point onward, everything proceeds according to normal physics.
A.
Th
e answer turns out
to be “a lot of things,” and they all happen very quickly, and it doesn’t end well
for the batter (or the pitcher). I sat down with some physics books, a Nolan Ryan action figure, and a bunch of videotapes of nuclear tests and tried to sort it all out. What follows is my best guess at a nanosecond-by-nanosecond portrait.
Th
e ball would be going so fast that everything else would be practically stationary. Even the molecules in the air would stand still. Air molecules would
vibrate back and forth at a few hundred miles per hour, but the ball would be moving through them at 600
million
miles per hour.
Th
is means that as far as the ball is concerned, they would just be hanging there, frozen.
Th
e ideas of aerodynamics wouldn’t apply here. Normally, air would flow around anything moving through it. But the air molecules in front of this ball wouldn’t have time to
be jostled out of the way.
Th
e ball would smack into them so hard that the atoms in the air molecules would actually fuse with the atoms in the ball’s surface. Each collision would release a burst of gamma rays and scattered particles.
1
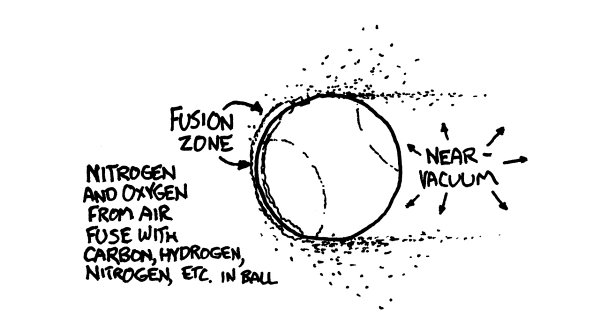
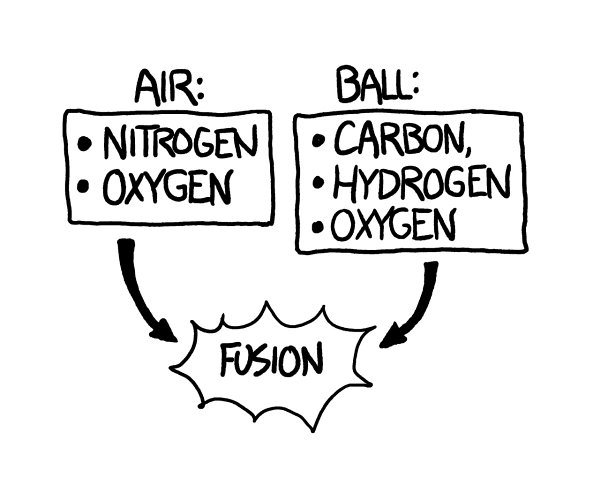
Th
ese gamma rays and debris would expand outward in a bubble centered on the pitcher’s mound.
Th
ey would start to tear apart the molecules in the air, ripping the electrons from the nuclei and turning the air in the stadium into an expanding bubble of incandescent plasma.
Th
e wall of this bubble would approach the batter at about the speed of light
—
only slightly ahead of the
ball itself.
Th
e constant fusion at the front of the ball would push back on it, slowing it down, as if the ball were a rocket flying tail-first while firing its engines. Unfortunately, the ball would be going so fast that even the tremendous force from this ongoing thermonuclear explosion would barely slow it down at all. It would, however, start to eat away at the surface, blasting tiny
fragments of the ball in all directions.
Th
ese fragments would be going so fast that when they hit air molecules, they would trigger two or three more rounds of fusion.
After about 70 nanoseconds the ball would arrive at home plate.
Th
e batter wouldn’t even have seen the pitcher let go of the ball, since the light carrying that information would arrive at about the same time the ball would.
Collisions with the air would have eaten the ball away almost completely, and it would now be a bullet-shaped cloud of expanding plasma (mainly carbon, oxygen, hydrogen, and nitrogen) ramming into the air and triggering more fusion as it went.
Th
e shell of x-rays would hit the batter first, and a handful of nanoseconds later the debris cloud would hit.
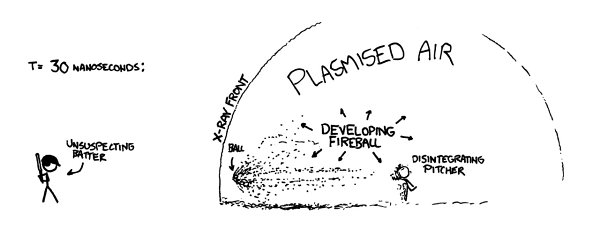
When it would reach home plate, the center of the cloud would still be moving at an appreciable fraction of the speed of light. It would hit the bat first, but then the batter, plate, and catcher would all be scooped up and carried backward through the backstop as they disintegrated.
Th
e shell of x-rays and superheated plasma would expand outward and upward, swallowing the backstop,
both teams, the stands, and the surrounding neighborhood
—
all in the first microsecond.
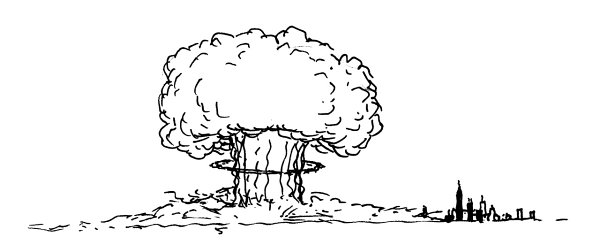
Suppose you’re watching from a hilltop outside the city.
Th
e first thing you would see would be a blinding light, far outshining the sun.
Th
is would gradually fade over the course of a few seconds, and a growing fireball would rise into a mushroom cloud.
Th
en, with a great roar, the blast wave would arrive,
tearing up trees and shredding houses.
Everything within roughly a mile of the park would be leveled, and a firestorm would engulf the surrounding city.
Th
e baseball diamond, now a sizable crater, would be centered a few hundred feet behind the former location of the backstop.
Major League Baseball Rule 6.08(b) suggests that in this situation, the batter would be considered “hit by pitch,” and would be eligible to advance to first base.
- 1
After I initially published this article, MIT physicist Hans Rinderknecht contacted me to say that he’d simulated this scenario on their lab’s computers. He found that early in the ball’s flight,
most of the air molecules were actually moving too quickly to cause fusion, and would pass right through the ball, heating it more slowly and uniformly than my original article described.
Spent Fuel Pool
Q.
What if I took a swim in a typical spent nuclear fuel pool? Would I need to dive to actually experience a fatal amount of radiation? How long could I stay safely at the surface?
—Jonathan Bastien-Filiatrault
A.
Assuming you’re a reasonably
good swimmer, you could probably survive treading water anywhere from 10 to 40 hours. At that point, you would black out from
fatigue and drown.
Th
is is also true for a pool without nuclear fuel in the bottom.
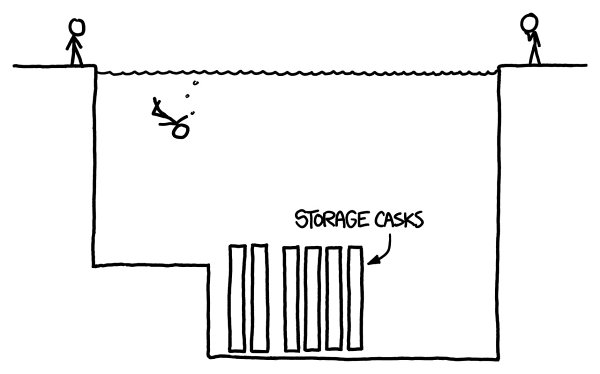
Spent fuel from nuclear reactors is highly radioactive. Water is good for both radiation shielding and cooling, so fuel is stored at the bottom of pools for a couple of decades until it’s inert enough to be moved into dry casks. We haven’t really agreed on where to put those dry casks yet. One of these days
we should probably figure that out.
Here’s the geometry of a typical fuel storage pool:
Th
e heat wouldn’t be a big problem.
Th
e water temperature in a fuel pool can in theory go as high as 50°C, but in practice it’s generally between 25°C and 35°C
—
warmer than most pools but cooler than a hot tub.
Th
e most highly radioactive fuel rods are those recently removed from a reactor. For the kinds of radiation coming off spent nuclear fuel, every 7 centimeters of water
cuts the amount of radiation in half. Based on the activity levels provided by Ontario Hydro in this report, this would be the region of danger for fresh fuel rods:
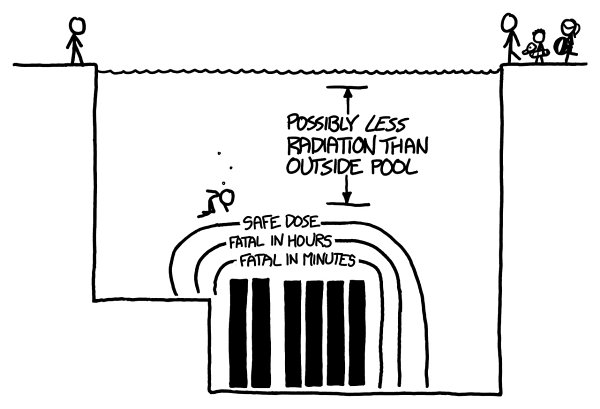
Swimming to the bottom, touching your elbows to a fresh fuel canister, and immediately swimming back up would probably be enough to kill you.
Yet outside the outer boundary, you could swim around as long as you wanted
—
the dose from the core would be less than the normal background dose you get walking around. In fact, as long as you were underwater, you would be shielded from
most of that normal background dose. You may actually receive a lower dose of radiation treading water in a spent fuel pool than walking around on the street.
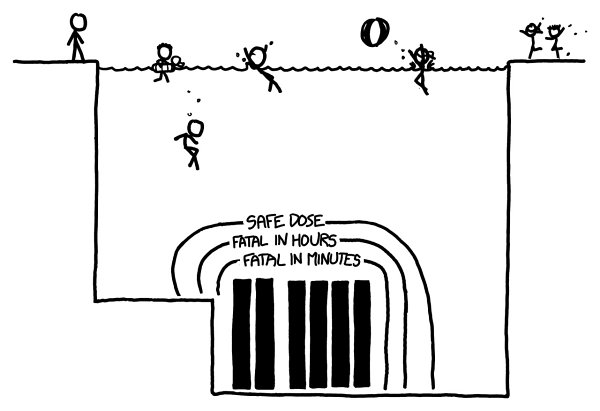
Remember: I am a cartoonist. If you follow my advice on safety around nuclear materials, you probably deserve whatever happens to you.
Th
at’s if everything goes as planned. If there’s corrosion in the spent fuel rod casings, there may be some fission products in the water.
Th
ey do a pretty good job of keeping the water clean, and it wouldn’t hurt you to swim in it, but it’s
radioactive enough that it wouldn’t be legal to sell it as bottled water.
1
We know spent fuel pools can be safe to swim in because they’re routinely serviced by human divers.
However, these divers have to be careful.
On August 31, 2010, a diver was servicing the spent fuel pool at the Leibstadt nuclear reactor in Switzerland. He spotted an unidentified length of tubing on the bottom
of the pool and radioed his supervisor to ask what to do. He was told to put it in his tool basket, which he did. Due to bubble noise in the pool, he didn’t hear his radiation alarm.
When the tool basket was lifted from the water, the room’s radiation alarms went off.
Th
e basket was dropped back in the water and the diver left the pool.
Th
e diver’s dosimeter badges showed that he’d received
a higher-than-normal whole-body dose, and the dose in his right hand was extremely high.
Th
e object turned out to be protective tubing from a radiation monitor in the reactor core, made highly radioactive by neutron flux. It had been accidentally sheared off while a capsule was being closed in 2006. It sank to a remote corner of the pool, where it sat unnoticed for four years.
Th
e tubing
was so radioactive that if he’d tucked it into a tool belt or shoulder bag, where it sat close to his body, he could’ve been killed. As it was, the water protected him, and only his hand
—
a body part more resistant to radiation than the delicate internal organs
—
received a heavy dose.
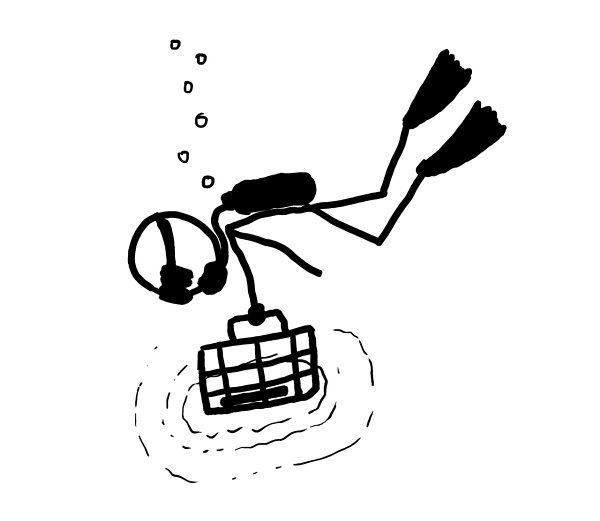
So, as far as swimming safety goes, the bottom line is that you’d probably be OK, as long as you didn’t dive to the bottom or pick up anything strange.
But just to be sure, I got in touch with a friend of mine who works at a research reactor, and asked him what he thought would happen to someone who tried to swim in their radiation containment pool.
“In
our
reactor?” He
thought about it for a moment. “You’d die pretty quickly, before reaching the water, from gunshot wounds.”
- 1
Which is too bad
—
it’d make a hell of an energy drink.
