1493: Uncovering the New World Columbus Created (41 page)
Read 1493: Uncovering the New World Columbus Created Online
Authors: Charles C. Mann
Tags: #Americas (North; Central; South; West Indies), #Expeditions & Discoveries, #United States, #Colonial Period (1600-1775), #History

The epicenter of what became known as “rubber fever” was Salem, Massachusetts, north of Boston. In 1825 a young Salem entrepreneur imported five hundred pairs of rubber shoes from Brazil. Ten years later, the number of imported shoes had grown to more than 400,000, about one for every forty Americans. Villagers in tiny hamlets at the mouth of the Amazon molded thousands of shoes to the dictates of Boston merchants. Garments impregnated with rubber were modern, high-tech, exciting—a perfect urban accessory. People flocked to stores.
The crash was inevitable. The idea of impermeable rubber boots and clothes was more exciting than the fact. Rubber simply didn’t work very well. In cold weather, the shoes became brittle; in hot weather, they melted. Boots placed in closets at the end of winter turned into black puddles by fall. The results smelled so bad that people found themselves burying their footgear in the garden. Daniel Webster, the senator and secretary of state, liked to tell the story of how he received a rubber cloak and hat as a gift. He wore them on a cold evening. By the time he reached his destination the cloak had become so rigid that he stood it in the street by the front door. Supposedly he propped the hat on top. “Some decorous gentlemen among us can also remember,” one critic wrote later, “that, in the nocturnal combats of their college days, a flinty India-rubber shoe, in cold weather, was a missive weapon of highly effective character.” Returned goods inundated rubber dealers. Public opinion swung violently against rubber.
Just before the collapse, in 1833, a bankrupt businessman named Charles Goodyear became interested in—and obsessed by—rubber. It was typical of Goodyear’s entrepreneurial acumen that he began to seek financial backing for a rubber venture just at the time investors were planning their exits from the field. A few weeks after Goodyear announced his intent to produce temperature-stable rubber he was thrown into debtor’s prison. In his cell he began work, mashing bits of rubber with a rolling pin. He was untroubled by any knowledge of chemistry but boundlessly determined. For years Goodyear wandered about the northeastern United States in a cloud of penury, trailed by his hungry wife and children, dodging bailiffs and pawning heirlooms. All the while he was mixing toxic chemicals, more or less randomly, in the hope that they would make rubber more stable. The Goodyears lived in an abandoned rubber factory in Staten Island. They lived in an abandoned rubber factory in Massachusetts. They lived in a shack in a Connecticut neighborhood called Sodom Hill (the name indicated its wholesomeness). They lived in a second abandoned rubber factory in Massachusetts. Sometimes the houses had no heat or food. Two of Goodyear’s children died.
Taking his cue from a dream told to him by another rubber obsessive, Goodyear began mixing rubber with sulfur. Nothing happened, he said later, until he accidentally dropped a lump of sulfur-treated rubber onto a wood stove. To his amazement, the rubber didn’t melt. The surface charred, but the inner material changed into a new kind of rubber that retained its shape and elasticity at high temperatures. Goodyear threw himself into reproducing the accident, a task impeded by his inability to afford any laboratory apparatus—he had to traipse from neighbor to neighbor, asking to use their wood stoves. Sometimes the sulfur process worked, sometimes it didn’t. Goodyear kept working, frustrated, hungry, haunted. When he was again thrown into debtor’s prison, he wrote to acquaintances from his cell, asking for supplies “to establish an India rubber factory for myself on the spot.” Eventually he borrowed money and paid the debt. A month later he was in another jail.
Along the way he befriended a young Englishman. Goodyear gave him a few of his successful samples and asked him to seek investors in Britain. By a circuitous path two thin, inch-and-a-half-long strips of Goodyear’s processed rubber ended up in the fall of 1842 at the laboratory of Thomas Hancock, a Manchester engineer who had developed processes for manipulating rubber. Hancock had no idea where these bits of rubber had originated. But he quickly realized that they didn’t melt in hot weather or become stiff in cold weather. The question was whether he could duplicate the accomplishment. It is unclear how much he was able to learn from Goodyear’s samples. Later he claimed to have “made no analysis of these little bits” from the other man—a remarkable demonstration of incuriosity, if true. In any case Hancock was more organized and knowledgeable than Goodyear and had better equipment. For a year and a half he systematically performed hundreds of small experiments. Eventually he, too, learned that immersing rubber in melted sulfur would transform it into something that would stay stretchy in cold weather and solid in hot weather. Later he called the process “vulcanization,” after the Roman god of fire. The British government granted Hancock a patent on May 21, 1844.
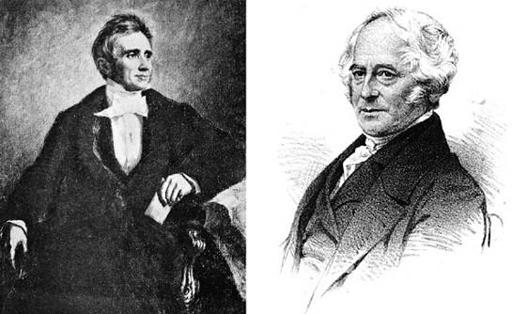
Identifying the inventor of the process of vulcanization, which makes rubber usable for industrial purposes, is complex. Charles Goodyear (left) had the basic idea first, but never fully understood the process; Thomas Hancock (right) patented the process before Goodyear and understood it better, but likely derived inspiration from seeing Goodyear’s initial samples. (
Photo credit 7.2
)
Three weeks later, the U.S. government awarded Goodyear
his
vulcanization patent. A glance at the patent shows that Goodyear never fully understood the process: a key ingredient, he claimed, was white lead, a metal-based pigment whose effect on rubber’s stability is “secondary, if anything,” according to E. Bryan Coughlin, of the Silvio O. Conte National Center for Polymer Research at the University of Massachusetts. “I’m not sure, because it’s not a standard treatment—maybe it has some catalytic effect.” By contrast, Coughlin told me, Hancock’s patent was “pretty straightforward.” Hancock stirred softened rubber into sulfur heated to 240°–250° F, just above its melting point. The longer he subjected it to heat, the more elasticity it lost. “That’s pretty much what I teach my students,” Coughlin said.
Goodyear didn’t understand the recipe for vulcanization, but he did understand that at last he had a business opportunity. Showing a previously unsuspected knack for publicity stunts, he spent $30,000 he did not have to create an entire room made of rubber for the Great Exhibition of 1851 at the Crystal Palace in London, the first world’s fair. Four years later he borrowed $50,000 more to display an even more lavish rubber room at the second world’s fair, the Exposition Universelle in Paris. Parisians lost their urban hauteur and gawped like rubes at Goodyear’s rubber vanity table, complete with rubber-framed mirror; arranged on the top was a battalion of rubber combs and rubber-handled brushes. In the center of the rubber floor was a hard rubber desk with a rubber inkwell and rubber pens. Rubber umbrellas stood at attention in a rubber umbrella-stand in the corner of two rubber walls, each decorated with paintings on rubber canvases. For weapons fans, there was a stand of knives in rubber sheaths, swords in rubber scabbards, and rifles with rubber stocks. Except for the unpleasant rubber smell, Goodyear’s exhibit was a triumph. “Napoleon III invested him with the Legion of Honor,” wrote the diplomat and historian Austin Coates, “and a Paris court sent him to prison for debt.” He received the medal in his cell. Goodyear was forced to sell some of his wife’s possessions to pay for their trip home. He died four years later, still awash in debt.
Afterward, Americans lionized Goodyear as a visionary. Books extolled him to children as an exemplar of the can-do spirit; a major tire company named itself after him. Meanwhile, Coates noted, “Hancock received English treatment: due respect while living, fading notice when dead, and on some suitable centenary thereafter, a postage stamp.”
Neither Goodyear nor Hancock had any idea
why
sulfur stabilized rubber—or why, for that matter, unadulterated rubber bounced and stretched. Nineteenth-century scientists found bouncing balls exactly as mystifying as sixteenth-century Spaniards. Stretch a thin hoop of iron: it will elongate slightly, then snap in two. A rubber band, by contrast, can stretch to three times its ordinary length, then return to its original shape. Why? And why did sulfur stop rubber from melting in the summer? “Nobody knew,” Coughlin told me. “It was a huge puzzle. And it was made harder by the fact that a lot of chemists didn’t really want to study it.”
The last half of the nineteenth century was a heady time for chemistry. Researchers were deciphering the underlying order of the physical world. They were placing the chemical elements into the periodic table, discovering the rules by which atoms combine into molecules, and learning that molecules could form regular crystals with structures that could be precisely identified.
Nowhere in these tidy intellectual schemes was a place for rubber. Chemists couldn’t make it form crystals. Worse, many standard chemical tests on rubber produced nonsensical answers. The analyses demonstrated that each rubber molecule was made up of carbon and hydrogen atoms. No problem there. But they also indicated that the carbon and hydrogen were piled up into jumbo-sized molecules made up of tens of thousands of atoms. To most chemists, this was absurd—molecules are the fundamental building blocks of chemical compounds, and no fundamental building block should be that big.
The obvious conclusion, chemists said, is that rubber must be a
colloid:
one or more compounds finely ground up and dispersed throughout other compounds. Glue is a colloid; so are peanut butter, bacon fat, and mud. Because colloids aren’t one substance but a mishmash of many different substances, they have no fundamental constituents. Looking for one would be like trying to find the molecular building blocks of a garbage heap. The chemistry of rubber was, one German researcher scoffed,
Schmierenchemie.
Literally,
Schmierenchemie
means “grease chemistry,” though Coughlin told me it might be better translated as “the chemistry of the gunk on the bottom of a test tube.”
Nonetheless, a few chemists ignored the disdain of their colleagues for rubber, prominent among them Hermann Staudinger, then at the Swiss Federal Institute of Technology in Zurich. A well-known researcher, he had already derived the chemical formulae for the basic flavors in coffee and pepper. (It is not unfair to charge Staudinger with inflicting instant coffee on the world.) Sometime during the First World War, he jumped into the entirely different field of rubber because of an intuitive belief that “high molecular compounds,” as he called them,
did
have basic building blocks, which were fantastically large molecules. Readers familiar with stories of successful scientific mavericks will not be surprised to learn that Staudinger attracted vehement opposition, that he kept piling up evidence for his hypothesis, and that the resistance grew irrational and vituperative. When he left Zurich to work at the University of Freiburg in 1925 he was denounced by colleagues during his farewell lecture. Presumably the antagonism was heightened by Staudinger’s penchant for picking fights. He once greeted the arrival of a rival’s book by gluing a denunciation—“This book is not a scientific work but propaganda”—onto the cover of the copy in his university library. In the end, though, Staudinger’s tale reached its denouement in the customary location: Stockholm, where he won the Nobel Prize for Chemistry in 1953.
Rubber and other elastomers, Staudinger showed, have molecules shaped like long chains.
2
“Long” is an accurate adjective: if a rubber molecule were as thick as a pencil, it would be as long as a football field. “Chain,” too, is accurate: all rubber molecules are made up of tens of thousands of identical, repeating links, each consisting of five carbon atoms and eight hydrogen atoms. The molecules of ordinary solid substances—the copper in a wire, say—are usually distributed in orderly arrays. Rubber molecules, by contrast, are higgledy-piggledy, the chains scrambled around each other in no discernible pattern. “The classic analogy is a bowl of spaghetti,” Coughlin explained to me. “But the analogy doesn’t really work unless you’re willing to say the noodles are a hundred yards long.” Stretching a rubber band pulls the tangled molecules into alignment, lining them up in parallel like strands of spaghetti in a box. As they unkink, the molecules go from a clumped snarl to their full length, which is why rubber can stretch. By contrast, the copper molecules in a wire are
already
lined up in an array, making it much harder for the material to lengthen—the difference is the difference between pulling the end of a loose, tangled string and trying to tug at a fully extended string. (The energy required to pull the chains straight is why rubber heats up when stretched.) As soon as the pressure is relaxed, the rubber molecules begin moving randomly, which naturally ensnarls them again; the rubber shrinks back to its original size.
When a lump of pure rubber is heated up, the rubber chains vibrate and slither around each other every which way and get even more chaotically disordered; the rubber loses whatever shape it has and turns into a puddle. Vulcanization prevents this. Immersing rubber in sulfur causes a chemical reaction in which rubber molecules link themselves together with chemical “bridges” formed of sulfur atoms. So ubiquitous are the bonds that a rubber band—a loop of vulcanized rubber—is actually a single, enormous, cross-linked molecule. With the molecules anchored together, they are more resistant to change: harder to align, harder to entangle, more resistant to extremes of temperature. Rubber suddenly becomes a stable material.
