Criminal Poisoning: Investigational Guide for Law Enforcement, Toxicologists, Forensic Scientists, and Attorneys (20 page)
Authors: John H. Trestrail

Conclusion
105
Figure 10-3
Figure 10-4
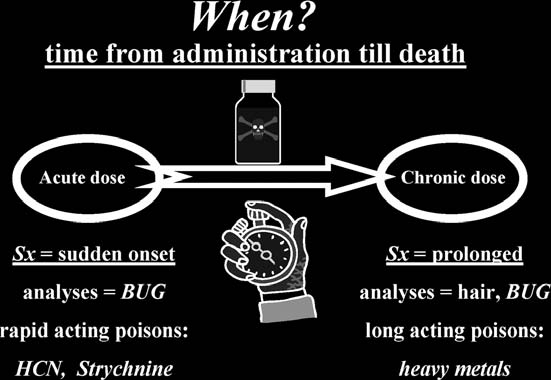
cyanide, strychnine). With a chronic dose situation, look for prolonged symptoms. Carry out analyses on the victim’s hair looking for heavy metals (e.g., arsenic, antimony, lead, thallium) (
see
Fig. 10-4
).
106
Criminal Poisoning
Figure 10-5
Figure 10-6
•
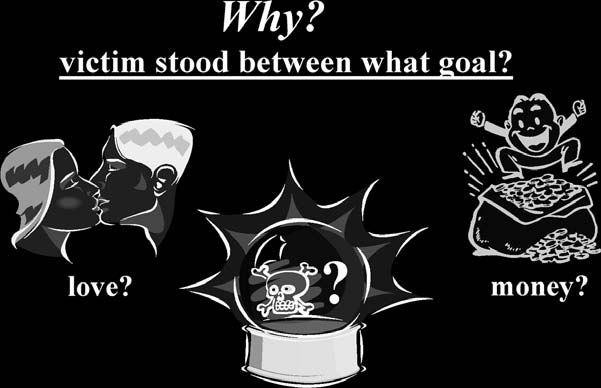
WHY
was the victim chosen? Did the victim stand between a goal that was so important to the offender that elimination of this individual led to obtaining the goal? (
see
Fig. 10-5
).
•
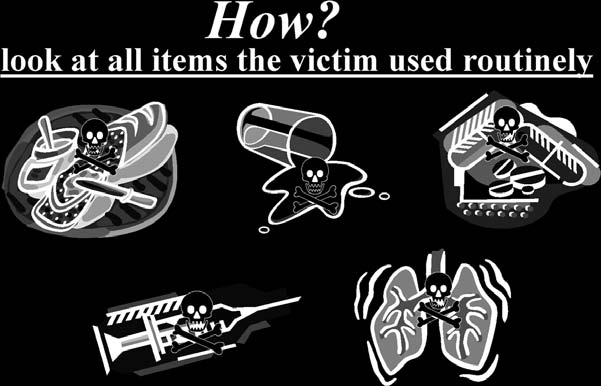
HOW
was the poison administered? Look at the items used routinely and solely by the victim (
see
Fig. 10-6
).
Conclusion
107
Figure 10-7
Figure 10-8
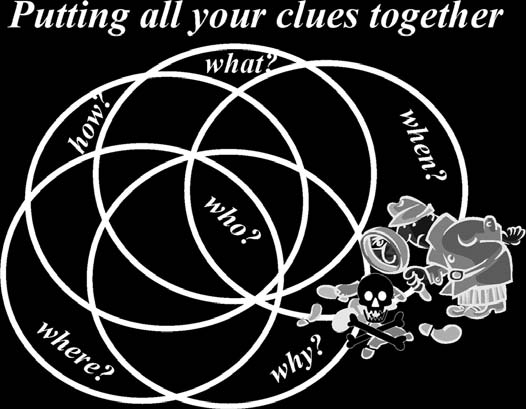
The clues provided by the answers to these questions will point to an individual who is a likely offender (
see
Fig. 10-7
). The bottom line is that if
investigators do not consider poisoning they will never detect it.
The entire purpose of this important reference work can be summarized
in the single graphic representation in
Fig. 10-8.
The results of our missing
108
Criminal Poisoning
Figure 10-9
deaths from poisoning over the years have resulted in a situation which is best depicted by
Fig. 10-9
. Think about it! And now
on the following page, a concluding poetic challenge, from the poisoner to the newly educated “Toxic Avengers.”
Conclusion
109
“The Poisoner”
by
John H. Trestrail III, RPh, FAACT, DABAT
The Borgias, DeMedicis and all those past—
you may have thought you had seen the last.
But, we poisoners are still around today.
And if you miss my crime, I’ll get away.
The body lies there neat and clean,
as the cause of death is seldom seen.
And the coroner may take time to pause—
“is this death due to a natural cause?”
An autopsy or tox screen may reveal death’s why,
but I hope the case will just slip by.
My crime is quiet and well thought through.
For you’re used to violence—can I fool you?
The event’s rarity is on my side.
For I count on you burying my homicide.
And though I roam free round the nation,
I live in fear of an exhumation.
The clues I leave may be hard to find,
you see, to me, I have a superior mind.
My weapons are there before your eyes,
but they are so very small—of molecular size.
I don’t think you’ll have a notion,
for mine is murder in slow motion.
It gives me time to just slip by,
and create my perfect alibi.
Where to look for me isn’t clear.
I may be far, or I may be near.
I could be a stranger, though it is quite rare,
for I’m probably related to the victim there.
I chose the place, the means, and time,
for poisoning is usually a household crime.
The knowledge gained by my living close,
made it so very easy to deliver the dose.
Seeing it as poisoning would be profound,
but I think you’ll miss it as you look around.
I’m a different kind of killer as you can see.
I am a POISONER—can you catch me?
Appendix
111
Appendix:
Some Common Homicidal Poisons
ANTIFREEZE (METHANOL [CH3-OH],
OR ETHYLENE GLYCOL [HO-CH2-CH2-OH])
Form:
•
Methanol (MeOH), also known as methyl alcohol or “wood alcohol,” is the simplest of the alcohols. For chronic alcoholics, this alcohol sometimes serves as a cheap substitute for ethanol (grain alcohol), as in the use of canned Sterno® as a source. Abuse of this toxic alcohol can have very dire consequences (e.g., blindness).
•
Ethylene glycol is chemically known as 1,2-ethanediol. It is a slightly vis-cous liquid.
Color:
•
Methanol: Colorless.
•
Ethylene glycol: Colorless.
Odor:
•
Methanol: Slight alcoholic odor.
•
Ethylene glycol: Odorless.
Solubility:
•
Methanol: Very water soluble.
•
Ethylene glycol: Very water soluble. It can absorb twice its weight in water.
Taste:
•
Methanol: A burning taste.
•
Ethylene glycol: Has a sweet taste, which has often led to the accidental ingestion of this substance by household pets.
From: Forensic Science and Medicine: Criminal Poisoning, Second Edition By: J. H. Trestrail, III © Humana Press Inc., Totowa, NJ
111
112
Common Homicidal Poisons
Source:
•
Methanol: Is a common ingredient in windshield-washing solutions, dupli-cating fluids, and paint removers and is commonly found in gas-line antifreeze, which may be 95% (v/v) methanol.
•
Ethylene glycol: Is commonly found in radiator antifreeze (in a concentration of ~95% [v/v]), and antifreeze products used in heating and cooling systems.
Lethal Dose:
•
Methanol: The fatal dose is estimated to be 30–240 mL (20–150 g).
•
Ethylene glycol: The approximate fatal dose of 95% ethylene glycol is estimated to be 1.5 mL/kg. For a person weighing 150 lb, this would be 102 mL
(3.5 fluid ounces).
How It Kills:
•
Methanol: The compound is oxidized in the body by the enzyme alcohol dehydrogenase into the more toxic compound formaldehyde (more commonly found in mortuary parlors), which in turn is further oxidized by aldehyde dehydrogenase to formic acid (the same compound that gives ants their sting).
•
Ethylene glycol: The compound is oxidized in the body by the enzyme alcohol dehydrogenase into the more toxic compound oxalic acid (a substance that serves as the common ingredient in some rust removers). This metabolite then combines with circulating calcium in the blood to form characteristic “envelope-shaped” (dihydrate) crystals of calcium oxalate, which can be found in the kidneys and urine. Needle-like crystals (monohydrate) can also be formed, which can lead to kidney damage.
Poison Notes:
None.
Victim of Antifreeze
Administered:
Both antifreeze substances can be easily administered in beverages that are expected to have a sweet or alcoholic taste.
Symptom Onset Time Interval:
•
Methanol: From a few hours to 30 h.
•
Ethylene glycol: From 4 to 12 h.
Symptoms—Acute:
•
Methanol: In the first few hours, the victim will appear inebriated and have gastritis. After a period of about 30 h, the victim will experience metabolic acidosis, visual disturbances, blindness, seizures, and coma, and death may occur. Patients have described the visual disturbances as being similar to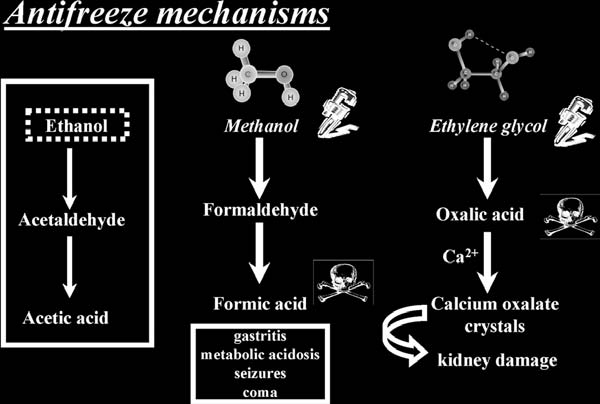
Appendix
