Ross & Wilson Anatomy and Physiology in Health and Illness (31 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

Platelets (thrombocytes)
These are very small non-nucleated discs, 2 to 4 μm in diameter, derived from the cytoplasm of megakaryocytes in red bone marrow (
Fig. 4.2
). They contain a variety of substances that promote blood clotting, which causes
haemostasis
(cessation of bleeding).
The normal blood platelet count is between 200 × 10
9
/l and 350 × 10
9
/l (200 000 to 350 000/mm
3
). The control of platelet production is not yet entirely clear but one stimulus is a fall in platelet count. The kidneys release a substance called
thrombopoietin
, which stimulates platelet synthesis.
The life span of platelets is between 8 and 11 days and those not used in haemostasis are destroyed by macrophages, mainly in the spleen. About a third of platelets are stored within the spleen rather than in the circulation; this is an emergency store that can be released as required to control excessive bleeding.
Haemostasis
When a blood vessel is damaged, loss of blood is stopped and healing occurs in a series of overlapping processes, in which platelets play a vital part. The more badly damaged the vessel wall is, the faster coagulation begins, sometimes as quickly as 15 seconds after injury.
1 Vasoconstriction
When platelets come into contact with a damaged blood vessel, their surface becomes sticky and they adhere to the damaged wall. They then release
serotonin
(5-hydroxytryptamine), which constricts (narrows) the vessel, reducing blood flow through it. Other chemicals that cause vasoconstriction, e.g. thromboxanes, are released by the damaged vessel itself.
2 Platelet plug formation
The adherent platelets clump to each other and release other substances, including
adenosine diphosphate
(ADP), which attract more platelets to the site. Passing platelets stick to those already at the damaged vessel and they too release their chemicals. This is a positive feedback system by which many platelets rapidly arrive at the site of vascular damage and quickly form a temporary seal – the platelet plug. Platelet plug formation is usually complete by 6 minutes after injury.
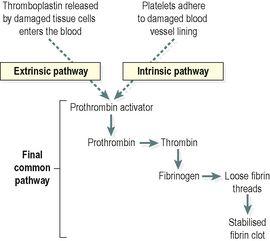
3 Coagulation (blood clotting)
This is a complex process that also involves a positive feedback system and only a few stages are included here. The factors involved are listed in
Table 4.3
. Their numbers represent the order in which they were discovered and not the order of participation in the clotting process. These clotting factors activate each other in a specific order, eventually resulting in the formation of
prothrombin activator
, which is the first step in the
final common pathway
. Prothrombin activates the enzyme
thrombin
, which converts inactive
fibrinogen
to insoluble threads of
fibrin
(
Fig. 4.12
). As clotting proceeds, the platelet plug is progressively stabilised by increasing amounts of fibrin laid down in a three-dimensional meshwork within it. The maturing blood clot traps blood cells and is much stronger than the rapidly formed platelet plug.
Table 4.3
Blood clotting factors
| I Fibrinogen II Prothrombin III Tissue factor (thromboplastin) IV Calcium (Ca V Labile factor, proaccelerin, Ac-globulin VII Stable factor, proconvertin VIII Antihaemophilic globulin (AHG), antihaemophilic factor A IX Christmas factor, plasma thromboplastin component (PTA), antihaemophilic factor B X Stuart Prower factor XI Plasma thromboplastin antecedent (PTA), antihaemophilic factor C XII Hageman factor XIII Fibrin stabilising factor |
| (There is no factor VI) |
| Vitamin K is essential for synthesis of factors II, VII, IX and X. |
Figure 4.12
Stages of blood clotting (coagulation).
The final common pathway can be initiated by two processes which often occur together: the extrinsic and intrinsic pathways (
Fig. 4.12
). The
extrinsic pathway
is activated rapidly (within seconds) following tissue damage. Damaged tissue releases a complex of chemicals called
thromboplastin
or tissue factor, which initiates coagulation. The
intrinsic pathway
is slower (3–6 minutes) and is triggered when blood comes into contact with damaged blood vessel lining (endothelium).
After a time the clot shrinks (
retracts
) because the platelets contract, squeezing out serum, a clear sticky fluid that consists of plasma from which clotting factors have been removed. Clot shrinkage pulls the edges of the damaged vessel together, reducing blood loss and closing off the hole in the vessel wall.
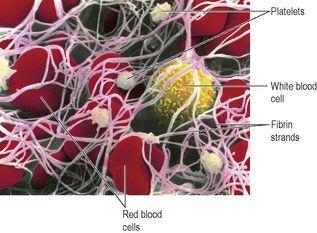
Figure 4.13
shows a scanning electron micrograph of a blood clot. The fibrin strands (pink) have trapped red blood cells, platelets and a white blood cell.
Figure 4.13
Scanning electron micrograph of a blood clot, showing the fibrin meshwork (pink strands), red blood cells, platelets and a white blood cell.
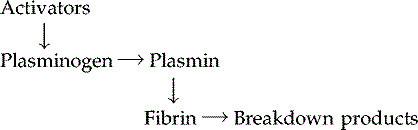
4 Fibrinolysis
After the clot has formed the process of removing it and healing the damaged blood vessel begins. The breakdown of the clot, or
fibrinolysis
, is the first stage. An inactive substance called
plasminogen
is present in the clot and is converted to the enzyme
plasmin
by activators released from the damaged endothelial cells. Plasmin initiates the breakdown of fibrin to soluble products that are treated as waste material and removed by phagocytosis. As the clot is removed, the healing process restores the integrity of the blood vessel wall.
Control of coagulation
The process of blood clotting relies heavily on several self-perpetuating processes – that is, once started, a positive feedback mechanism promotes their continuation. For example, thrombin is a powerful stimulator of its own production. The body therefore possesses several mechanisms to control and limit the coagulation cascade; otherwise once started the clotting process would spread throughout the circulatory system, far beyond requirements. The main controls are:
•
the perfect smoothness of normal blood vessel lining means that platelets do not adhere to it
•
the binding of thrombin to a special thrombin receptor on the cells lining blood vessels; once bound, thrombin is inactivated
•
the presence of natural anticoagulants, e.g. heparin, in the blood, which inactivate clotting factors.
Erythrocyte disorders
Learning outcomes
After studying this section, you should be able to:
define the term anaemia
compare and contrast the causes and effects of iron deficiency, megaloblastic, aplastic, hypoplastic and haemolytic anaemias