Ross & Wilson Anatomy and Physiology in Health and Illness (14 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

The body needs chemical energy to:
•
drive synthetic reactions (i.e. building biological molecules)
•
fuel movement (locomotion)
•
transport substances across membranes.
Enzymes
Many of the body’s chemical reactions can be reproduced in a test-tube. Surprisingly, the rate at which the reactions then occur usually plummets to the extent that, for all practical purposes, chemical activity ceases. The cells of the body have developed a solution to this apparent problem – they are equipped with a huge array of enzymes. Enzymes are proteins that act as
catalysts
for biochemical reactions – that is, they speed the reaction up but are not themselves changed by it, and therefore can be used over and over again. Enzymes are very selective and will usually catalyse only one specific reaction. The molecule(s) entering the reaction is called the
substrate
and it binds to a very specific site on the enzyme, called the
active site
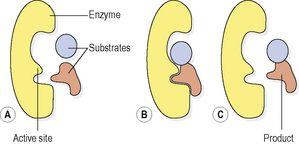
. Whilst the substrate(s) is bound to the active site the reaction proceeds, and once it is complete the product(s) of the reaction breaks away from the enzyme and the active site is ready for use again (
Fig. 2.11
).
Figure 2.11
Action of an enzyme: A.
Enzyme and substrates.
B.
Enzyme–substrate complex.
C.
Enzyme and product.
Enzyme action is reduced or stopped altogether if conditions are unsuitable. Increased or decreased temperature is likely to reduce activity, as is any change in pH. Some enzymes require the presence of a
cofactor
, an ion or small molecule that allows the enzyme to bind its substrate(s). Some vitamins are cofactors in enzyme reactions.
Enzymes can catalyse both synthetic and breakdown reactions, and their names (almost always!) end in ˜ase. When an enzyme catalyses the combination of two or more substrates into a larger product, this is called an
anabolic reaction
.
Catabolic reactions
involve the breakdown of the substrate into smaller products, as occurs during the digestion of foods.
Movement of substances within body fluids
Learning outcomes
After studying this section, you should be able to:
compare and contrast the processes of osmosis and diffusion
using these concepts, describe how molecules move within and between body compartments.
Movement of substances within and between body fluids, sometimes across a barrier such as the cell membrane, is essential in normal physiology.
From a physical point of view, substances will always travel from an area of high concentration to one of low concentration, assuming that there is no barrier in the way. Between two such areas, there exists a
concentration gradient
and movement of substances occurs
down
the concentration gradient, or downhill, until concentrations on each side are equal (
equilibrium
is reached). No energy is required for such movement, so this process is described as
passive
.

There are many examples in the body of substances moving
uphill
, i.e. against the concentration gradient; in this case, chemical energy is required, usually in the form of ATP. These processes are described as
active
. Movement of substances across cell membranes by active transport is described on
page 32
.
Passive movement of substances in the body proceeds usually in one of two main ways –
diffusion
or
osmosis
.
Diffusion
Diffusion refers to the movement of a chemical substance from an area of high concentration to an area of low concentration, and occurs mainly in gases, liquids and solutions. Sugar molecules heaped at the bottom of a cup of coffee that has not been stirred will, in time, become evenly distributed throughout the liquid by diffusion (
Fig. 2.12
). The process of diffusion is speeded up if the temperature rises and/or the concentration of the diffusing substance is increased.
Figure 2.12
The process of diffusion:
a spoonful of sugar in a cup of coffee.
Diffusion can also occur across a semipermeable membrane, such as the plasma membrane or the capillary wall. Only molecules able to cross the membrane will be able to diffuse through. For example, oxygen diffuses freely through the walls of the alveoli (airsacs in the lungs), where oxygen concentrations are high, into the bloodstream, where oxygen concentrations are low. However, blood cells and large protein molecules in the plasma are too large to cross and so remain in the blood.
Osmosis
While diffusion of solute molecules across a semipermeable membrane results in equal concentrations of the solute on both sides of the membrane,
osmosis
refers specifically to diffusion of water down its concentration gradient. This is usually because the solute molecules are too large to pass through the pores in the membrane. The force with which this occurs is called the
osmotic pressure
. Imagine two solutions of sugar separated by a semipermeable membrane whose pores are too small to let the sugar molecules through. On one side, the sugar solution is twice as concentrated as on the other. After a period of time, the concentration of sugar molecules will have equalised on both sides of the membrane, not because sugar molecules have diffused across the membrane, but because osmotic pressure across the membrane pulls water from the dilute solution into the concentrated solution – i.e. water has moved down its concentration gradient. Osmosis proceeds until equilibrium is reached, at which point the solutions on each side of the membrane are of the same concentration and are said to be
isotonic
. The importance of careful control of solute concentrations in the body fluids can be illustrated by looking at what happens to a cell (e.g. a red blood cell) when it is exposed to solutions that differ from normal physiological conditions.