Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy
Ross & Wilson Anatomy and Physiology in Health and Illness (12 page)

Table 2.3
Examples of normal plasma levels
| Substance | Amount in moles | Amount in other units |
|---|---|---|
| Chloride | 97–106 mmol/l | 97–106 mEq/l * |
| Sodium | 135–143 mmol/l | 135–143 mEq/l * |
| Glucose | 3.5–5.5 mmol/l | 60–100 mg/100 ml |
| Iron | 14–35 mmol/l | 90–196 mg/100 ml |
Acids, alkalis and pH
The concentration of hydrogen ions ([H
+
]) in a solution is a measure of the acidity of the solution. Control of hydrogen ion levels in body fluids is an important factor in maintaining a stable internal environment.
An acid substance releases hydrogen ions when in solution. On the other hand, a basic (alkaline) substance accepts hydrogen ions, often with the release of hydroxyl (OH
−
) ions. A salt releases other anions and cations when dissolved; sodium chloride is therefore a salt because in solution it releases sodium and chloride ions.
*
Milliequivalents per litre (mEq/l)

Concentration is expressed as:
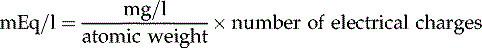
The pH scale
The standard scale for measurement of hydrogen ion concentration in solution is the pH scale. The scale measures from 0 to 14, with 7, the midpoint, as neutral; this is the pH of water. Water is a neutral molecule, neither acid nor alkaline, because when the molecule breaks up into its constituent ions, it releases one H
+
and one OH
−
, which balance one another. Most body fluids are close to neutral, because strong acids and bases are damaging to living tissues, and body fluids contain
buffers
, themselves weak acids and bases, to keep their pH within narrow ranges.
A pH reading below 7 indicates an
acid solution
, while readings above 7 indicate
alkalinity
(
Fig. 2.6
). A change of one whole number on the pH scale indicates a 10-fold change in [H
+
]. Therefore, a solution of pH 5 contains ten times as many hydrogen ions as a solution of pH 6.
Figure 2.6
The pH scale.
Not all acids ionise completely when dissolved in water. The hydrogen ion concentration is, therefore, a measure of the amount of
dissociated acid
(ionised acid) rather than of the total amount of acid present. Strong acids dissociate more freely than weak acids, e.g. hydrochloric acid dissociates freely into H
+
and Cl
−
, while carbonic acid dissociates much less freely into H
+
and HCO
3
−
.
Likewise, not all bases dissociate completely. Strong bases dissociate more fully, i.e. release more OH
−
than weaker ones.
pH values of the body fluids
The pH of body fluids must be maintained within relatively narrow limits depending on the fluid concerned. The normal range of pH values of some body fluids are shown in
Table 2.4
.
Table 2.4
pH values of certain body fluids
| Body fluid | pH |
|---|---|
| Blood | 7.35 to 7.45 |
| Saliva | 5.4 to 7.5 |
| Gastric juice | 1.5 to 3.5 |
| Bile | 6 to 8.5 |
| Urine | 4.5 to 8.0 |
The highly acid pH of the gastric juice is maintained by hydrochloric acid secreted by the parietal cells in the walls of the gastric glands. The low pH of the stomach fluids destroys microbes and toxins that may be swallowed in food or drink. Saliva has a pH of between 5.4 and 7.5, which is the optimum value for the action of salivary amylase, the enzyme present in saliva which initiates the digestion of carbohydrates. Amylase is destroyed by gastric acid when it reaches the stomach.
Blood pH is kept between 7.35 and 7.45, and outwith this narrow range there is severe disruption of normal physiological and biochemical processes. Normal metabolic activity of body cells produces certain acids and alkalis, which would tend to alter the pH of the tissue fluid and blood. Chemical
buffers
are responsible for keeping body pH stable.
Buffers
Despite the constant cellular production of acid and alkaline substances, body pH is kept stable by systems of buffering chemicals in body fluids and tissues. These buffering mechanisms temporarily neutralise fluctuations in pH, but can function effectively only if there is some means by which excess acid or alkali can be excreted from the body. The organs most active in this way are the
lungs
and the
kidneys
. The lungs are important regulators of blood pH because they excrete carbon dioxide (CO
2
). CO
2
increases [H
+
] in body fluids because it combines with water to form carbonic acid, which then dissociates into a bicarbonate ion and a hydrogen ion.

The lungs, therefore, help to control blood pH by regulating levels of excreted CO
2
. The brain detects rising [H
+
] in the blood and stimulates breathing, causing increased CO
2
loss and a fall in [H
+
]. Conversely, if blood pH becomes too alkaline, the brain can reduce the respiration rate to increase CO
2
levels and increase [H
+
], restoring pH towards normal (see
Ch. 10
).
The kidneys regulate blood pH by increasing or decreasing the excretion of hydrogen and bicarbonate ions as required. If pH falls, hydrogen ion excretion is increased and bicarbonate conserved; the reverse happens if pH rises. In addition, the kidneys generate bicarbonate ions as a by-product of amino acid breakdown in the renal tubules; this process also generates ammonium ions, which are rapidly excreted.
Other buffer systems include body proteins, which absorb excess H
+
, and phosphate, which is particularly important in controlling pH inside cells. The buffer and excretory systems of the body together maintain the
acid–base balance
so that the pH range of the blood remains within normal, but narrow, limits.
Acidosis and alkalosis
The buffer systems described above compensate for most pH fluctuations, but these reserves are limited and, in extreme cases, can become exhausted. When the pH falls below 7.35, and all the reserves of alkaline buffers are used up, the condition of
acidosis
exists. In the reverse situation, when the pH rises above 7.45, the increased alkali uses up all the acid reserve and the state of
alkalosis
exists.
Acidosis and alkalosis are both dangerous, particularly to the central nervous system and the cardiovascular system. In practice, acidotic conditions are commoner than alkalotic ones, because the body tends to produce more acid than alkali. Acidosis may follow respiratory problems, if the lungs are not excreting CO
2
as efficiently as normal, or if the body is producing excess acids (e.g. diabetic ketoacidosis,
p. 228
) or in kidney disease, if renal H
+
excretion is reduced. Alkalosis may be caused by loss of acidic substances through vomiting, diarrhoea, endocrine disorders or diuretic therapy, which stimulates increased renal excretion. Rarely, it may follow increased respiratory effort, such as in an acute anxiety attack where excessive amounts of CO
2
are lost through overbreathing (hyperventilation).
Important biological molecules
Learning outcomes



