Ross & Wilson Anatomy and Physiology in Health and Illness (10 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

Because living tissues are composed of chemical building blocks, the study of anatomy and physiology depends upon some understanding of biochemistry, the chemistry of life. This chapter introduces core concepts in chemistry that will underpin the remaining chapters in this book.
Atoms, molecules and compounds
Learning outcomes
After studying this section, you should be able to:
define the following terms: atomic number, atomic weight, isotope, molecular weight, ion, electrolyte, pH, acid and alkali
describe the structure of an atom
discuss the types of bond that hold molecules together
outline the concept of molar concentration
explain the importance of buffers in the regulation of pH.
The
atom
is the smallest unit of an element that exists as a stable entity. An
element
is a substance containing only one type of atom, e.g. iron contains only iron atoms. When a substance contains two or more different types of atom, it is called a
compound
. For instance, water is a compound containing both hydrogen and oxygen atoms.
There are 92 naturally occurring elements, but the wide variety of compounds that make up living tissues are composed almost entirely of only four: carbon, hydrogen, oxygen and nitrogen. Small amounts (about 4% of body weight) of others are present, including sodium, potassium, calcium and phosphorus.
Atomic structure
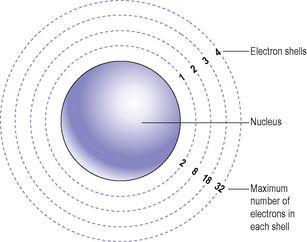
Atoms are mainly empty space, with a tiny central nucleus containing
protons
and
neutrons
surrounded by clouds of tiny orbiting
electrons
(
Fig. 2.1
). Neutrons carry no electrical charge, but protons are positively charged, and electrons are negatively charged. Because atoms contain equal numbers of protons and electrons, they carry no net charge.
Figure 2.1
The atom, showing the nucleus and four electron shells.
These subatomic particles differ also in terms of their mass. Electrons are so small that their mass is negligible, but the bigger neutrons and protons carry one atomic mass unit each. The physical characteristics of electrons, protons and neutrons are summarised in
Table 2.1
.
Table 2.1
Characteristics of subatomic particles
| Particle | Mass | Electric charge |
|---|---|---|
| Proton | 1 unit | 1 positive |
| Neutron | 1 unit | neutral |
| Electron | negligible | 1 negative |
Atomic number and atomic weight
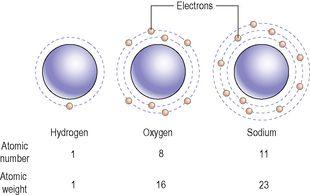
What makes one element different from another is the number of protons in the nuclei of its atoms (
Fig. 2.2
). This is called the
atomic number
and each element has its own atomic number, unique to its atoms. For instance, hydrogen has only one proton per nucleus, oxygen has eight and sodium has eleven. The atomic numbers of hydrogen, oxygen and sodium are therefore 1, 8 and 11 respectively. The
atomic weight
of an element is the sum of the protons and neutrons in the atomic nucleus.
Figure 2.2
The atomic structures of the elements hydrogen, oxygen and sodium.
The electrons are shown in
Figure 2.1
as though they orbit in concentric rings round the nucleus. These shells diagrammatically represent the different energy levels of the electrons in relation to the nucleus, not their physical positions. The first energy level can hold only two electrons and is filled first. The second energy level can hold only eight electrons and is filled next. The third and subsequent energy levels hold increased numbers of electrons, each containing more than the preceding level.
The
electron configuration
describes the distribution of the electrons in each element, e.g. sodium is 2 8 1 (
Fig. 2.2
).
The chemistry of life depends upon the ability of atoms to react and combine with one another, to produce the wide range of molecules required for biological diversity. The atomic particles important for this are the electrons of the outermost shell. An atom is reactive when it does not have a stable number of electrons in its outer shell, and may donate, receive or share electrons with one or more other atoms to achieve stability. This will be described more fully in the section discussing molecules and compounds.
Isotopes
These are atoms of an element in which there is a
different number of neutrons in the nucleus
. This does not affect the electrical activity of these atoms because neutrons carry no electrical charge, but it does affect their atomic weight. For example, there are three forms of the hydrogen atom. The most common form has one proton in the nucleus and one orbiting electron. Another form (
deuterium
) has one proton and one neutron in the nucleus. A third form (
tritium
) has one proton and two neutrons in the nucleus and one orbiting electron. These three forms of hydrogen are called
isotopes
(
Fig. 2.3
).