Ross & Wilson Anatomy and Physiology in Health and Illness (13 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

After studying this section, you should be able to:
describe in simple terms the chemical nature of sugars, proteins, lipids, nucleotides and enzymes
discuss the biological importance of each of these important groups of molecules.
Carbohydrates
Carbohydrates (sugars and starches) are composed of carbon, oxygen and hydrogen. The carbon atoms are normally arranged in a ring, with the oxygen and hydrogen atoms linked to them. The structures of glucose, fructose and sucrose are shown in
Figure 2.7
. When two sugars combine to form a bigger sugar, a water molecule is expelled and the bond formed is called a
glycosidic linkage
.
Figure 2.7
The combination of glucose and fructose to make sucrose.
Glucose, the main form in which sugar is used by cells, is a monosaccharide. Monosaccharides can be linked together to form bigger sugars, ranging in size from two sugar units (
disaccharides
), e.g. sucrose (
Fig. 2.7
) or table sugar, to long chains containing many thousands of monosaccharides e.g. starch. Such complex carbohydrates are called
polysaccharides
.
Glucose can be broken down (metabolised) in either the presence (
aerobically
) or the absence (
anaerobically
) of oxygen, but the process is much more efficient when O
2
is used. During this process, energy, water and carbon dioxide are released (
p. 307
). To ensure a constant supply of glucose for cellular metabolism, blood glucose levels are tightly controlled. The sugars:
•
provide a ready source of energy to fuel cell metabolism (
p. 307
)
•
provide a form of energy storage, e.g. glycogen (
p. 307
)
•
form an integral part of the structure of DNA and RNA (
pp. 429
,
431
)
•
can act as receptors on the cell surface, allowing the cell to recognise other molecules and cells.
Amino acids and proteins
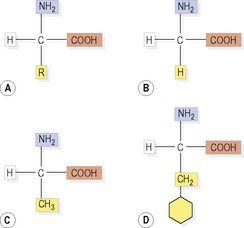
Amino acids always contain carbon, hydrogen, oxygen and nitrogen, and many in addition carry sulphur. In human biochemistry, 20 amino acids are used as the principal building blocks of protein, although there are others; for instance, there are some amino acids used only in certain proteins, and some are seen only in microbial products. Of the amino acids used in human protein synthesis, there is a basic common structure, including an amino group (NH
2
), a carboxyl group (COOH) and a hydrogen atom. What makes one amino acid different from the next is a variable side chain. The basic structure and three common amino acids are shown in
Figure 2.8
. As in formation of glycosidic linkages, when two amino acids join up the reaction expels a molecule of water and the resulting bond is called a
peptide bond
.
Figure 2.8
Amino acid structures: A.
Common structure, R = variable side chain.
B.
Glycine, the simplest amino acid.
C.
Alanine.
D.
Phenylalanine.
Proteins are made from amino acids joined together, and are the main family of molecules from which the human body is built. Protein molecules vary enormously in size, shape, chemical constituents and function. Many important groups of biologically active substances are proteins, e.g.:
•
carrier molecules, e.g. haemoglobin (
p. 59
)
•
enzymes (
p. 24
)
•
many hormones, e.g. insulin (
p. 218
)
•
antibodies (
pp. 371–372
).
Proteins can also be used as an alternative energy source, usually in starvation. In starvation, the main source of body protein is muscle tissue, so it is accompanied by wasting of muscles.
Lipids
The lipids are a diverse group of substances whose common property is an inability to mix with water (i.e. they are
hydrophobic
). They are made up of carbon, hydrogen and oxygen atoms. The most important groups of lipids include:
•
phospholipids
, integral to cell membrane structure. They form a double layer, providing a water-repellant barrier separating the cell contents from its environment (
p. 28
)
•
certain vitamins (
p. 270
). The fat-soluble vitamins are A, D, E and K
•
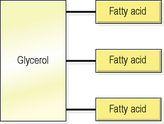
fats (triglycerides)
, stored in adipose tissue (
p. 37
) as an energy source. Fat also insulates the body and protects internal organs. A molecule of fat contains three fatty acids attached to a molecule of glycerol (
Fig. 2.9
). When fat is broken down under optimal conditions, more energy is released than when glucose is fully broken down.
Figure 2.9
Structure of a fat (triglyceride) molecule.
Fats are classified as
saturated
or
unsaturated
, depending on the chemical nature of the fatty acids present. Saturated fat tends to be solid, whereas unsaturated fats are fluid.
•
prostaglandins
are important chemicals derived from fatty acids and are involved in inflammation (
p. 367
) and other processes.
•
cholesterol
is a lipid made in the liver and available in the diet (
p. 269
). It is an integral part of cell membranes and is used to make steroid hormones (
p. 216
).
Nucleotides
Nucleic acids
These are the largest molecules in the body and are built from nucleotides. They include deoxyribonucleic acid (DNA,
p. 429
) and ribonucleic acid (RNA,
p. 431
).
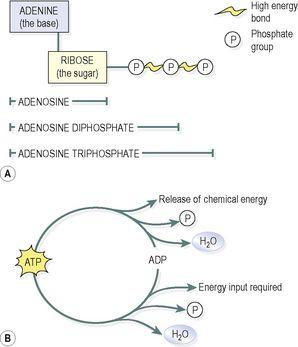
Adenosine triphosphate (ATP)
ATP is a nucleotide that contains ribose (the sugar unit), adenine (the base) and three phosphate groups attached to the ribose (
Fig. 2.10A
). It is sometimes known as the energy currency of the body, which implies that the body has to ‘earn’ (synthesise) it before it can ‘spend’ it. Many of the body’s huge number of reactions release energy, e.g. the breakdown of sugars in the presence of O
2
. The body captures the energy released by these reactions, using it to make ATP from adenosine diphosphate (ADP). When the body needs chemical energy to fuel cellular activities, ATP releases its stored energy, water and a phosphate group through the splitting of a high-energy phosphate bond, and reverts to ADP (
Fig. 2.10B
).
Figure 2.10
ATP and ADP: A.
Structures.
B.
Conversion cycle.