The Washington Manual Internship Survival Guide (14 page)
Read The Washington Manual Internship Survival Guide Online
Authors: Thomas M. de Fer,Eric Knoche,Gina Larossa,Heather Sateia
Tags: #Medical, #Internal Medicine

Evaluation
•
A careful H&P should be done, paying close attention to fluid status and the neurologic examination.
•
Plasma osmolality, urine osmolality, and urine [Na
+
] should be measured.
•
Solute excretion rate = urine osmolality × urine volume.
•
Refer
Figure 17-2
.
Treatment
•
Underlying conditions should be treated (e.g., hyperglycemia, diarrhea).
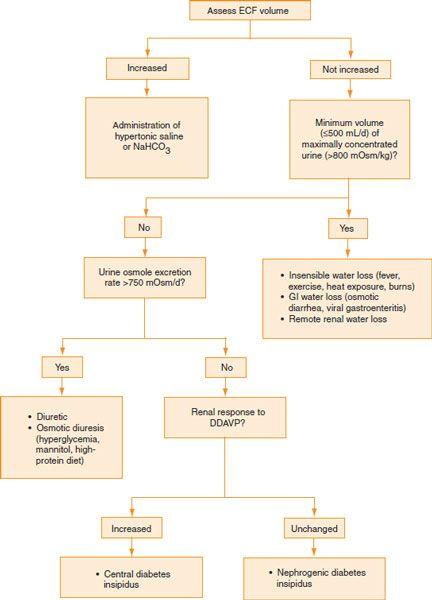
Figure 17-2.
Evaluation of hypernatremia.
•
ECF volume should be restored in hypovolemic patients with isotonic saline.
Free water deficit (L) = (plasma [Na
+
] − 140)/140 × TBW (L) TBW = 0.6 × body weight (kg)
•
As with hyponatremia,
correcting hypernatremia too rapidly is potentially dangerous
. The rate of correction of the plasma [Na
+
] should not exceed 0.5 mmol/L/h and the [Na
+
] should decrease by
no more than 12 mmol/L over the first 24 hours. With chronic asymptomatic hypernatremia, lower [Na
+
] more slowly, about 5–8 mmol/L/d.
•
Don’t forget to take into account ongoing losses. The safest route is PO or NG tube administration of water. Alternatively, ½NS (0.45%), ¼NS (0.225%), or D5W can be given IV. Reassess volume status and [Na
+
] every 8 to 12 hours.
•
Central diabetes insipidus is treated with intranasal dDAVP.
•
Nephrogenic diabetes insipidus may be reversible by treating the underlying disorder or eliminating the offending drug (e.g., lithium).
Hypokalemia
•
Defined as a [K
+
] <3.5 mmol/L, the clinical features vary greatly. Myalgias and weakness are common complaints.
•
Severe hypokalemia can result in an increased risk of arrhythmias. The [K
+
] level of cardiac patients is generally maintained above 4 mmol/L.
Etiology
•
Decreased intake
: This is infrequently the sole cause but can exacerbate other causes of hypokalemia.
•
Transcellular shifts
: Metabolic alkalosis, insulin, stress-induced catecholamine release, β-adrenergic agonists, and anabolic states all cause K
+
to shift into cells.
•
Nonrenal and renal loss
: Renal loss of K
+
may be caused by increased distal K
+
secretion (mineralocorticoid excess, Liddle’s syndrome) or increased distal tubular flow rate (loop and thiazide diuretics, osmotic diuresis, Bartter and Gitelman syndromes). GI causes include vomiting and diarrhea. Coexistent
hypomagnesemia
should be ruled out.
Evaluation
•
When the etiology of hypokalemia is not immediately apparent, renal K
+
excretion and acid–base status can help identify the cause.
•
The transtubular potassium gradient is calculated as follows:
TTKG = (U
[K+]
/P
[K+]
)/(U
osm
/P
osm
)
• TTKG <2 suggests a nonrenal source of K
+
loss.
• TTKG >4 suggests inappropriate renal K
+
secretion.
•
Acid–base status: Hypokalemia is generally associated with metabolic alkalosis. The finding of metabolic acidosis implies lower GI losses, distal RTA, or DKA.
Treatment
•
K
+
may be repleted either orally or IV. It is difficult to provide an algorithmic approach to replacing K
+
as
the degree of total depletion does not correlate well with plasma levels.
•
It is generally safer and more cost-effective to replace K
+
via the oral route.
Caution should be used in replacing
+
in patients with renal insufficiency
. A reasonable estimate in patients with normal renal function is that every 10 mmol of KCl will increase the serum level by 0.05 to 0.1 mmol/L.
•
Severe hypokalemia or patients who cannot take anything PO should be treated with IV KCl.
The rate of infusion should not exceed 10 mmol/h for a peripheral line
. Rates higher than this should be administered via a central line and rates >20 mmol/h require observation unit/ICU monitoring. Rates >40 mmol/h should only occur in the ICU for patients with life-threatening hypokalemia.
•
If
hypomagnesemia
is present, this should be supplemented as well.
Hyperkalemia
•
Defined as a [K
+
] >5 mmol/L, the most serious effect is cardiac toxicity. Toxicity also depends on the acuity of the hyperkalemia. Gradually developing, chronic, modest hyperkalemia in the range of 5.0 to 5.6 mmol/L is generally tolerated and does not necessarily require aggressive treatment.
•
If a suspicious result is received, consider repeating it stat; [K
+
] can be obtained on an ABG.
•
An ECG must be obtained. Refer to ECG interpretation section in Chapter 19. Look for peaked T waves, prolonged PR interval, and QRS duration. Consider continuous cardiac monitoring.
Etiology
•
Pseudohyperkalemia
is caused by K
+
movement out of cells associated with venipuncture. This may be seen with repeated fist clenching, prolonged tourniquet time, hemolysis, leukocytosis, or thrombocytosis.
•
Increased K
+
intake
is an unusual cause of hyperkalemia but may be seen in the setting of excess K
+
replacement, renal insufficiency, or both.
•
Transcellular shifts
: Acidosis, insulin deficiency, drugs (e.g., succinylcholine, β-blockers), hypertonicity (e.g., hyperglycemia), hemolysis, tumor lysis, rhabdomyolysis, and hyperkalemic periodic paralysis all cause potassium to shift out of cells.
•
Decreased renal K
+
excretion
is a common cause of chronic hyperkalemia. Major causes of decreased renal potassium excretion include
• Acute or chronic kidney disease
• Decreased effective circulating volume
• Hypoaldosteronism
▪ Primary adrenal insufficiency
▪ Hyporeninemic hypoaldosteronism (type 4 RTA)
• Drugs (e.g., NSAIDs, ACE inhibitors, angiotensin receptor blockers, cyclosporine, heparin, spironolactone, triamterene, amiloride, trimethoprim, pentamidine)
Evaluation
•
Rule out pseudohyperkalemia by repeating the serum electrolytes. Consider drawing the sample without the use of a tourniquet or fist clenching.
•
Obtain a stat ECG and an ABG (if acidosis is a concern).
•
Assess the patient’s urine output and renal function.
•
Examine the patient, paying particular attention to ECF volume status.
•
Review the patient’s medication list.
•
Assessment of TTKG and plasma renin and aldosterone levels may be useful when etiology is not immediately apparent.
• TTKG > 10 suggests that renal tubular mechanisms for K
+
secretion are intact. In this case, hyperkalemia could be due to high K
+
intake and/or decreased effective circulating volume causing diminished distal solute delivery and hence decreased K
+
secretion.
• TTKG < 7 implies impaired K
+
secretion caused by hypoaldosteronism, aldosterone resistance, or hyporeninemic hypoaldosteronism.
Treatment
•
Stop all exogenous K
+
and potentially offending drugs.
•
Not all hyperkalemia requires immediate aggressive treatment. This is particularly true of CKD/ESRD patients who often have mild hyperkalemia (5.0 to 5.6 mmol/L). Unless there are clinical signs of significant hyperkalemia, the acute management below is usually unnecessary.
•
Severe hyperkalemia or hyperkalemia with ECG changes requires emergent treatment. Do not do this by yourself. Call your resident immediately
.
•
Acute treatment
•
Calcium gluconate
10%, 10 mL IV over 2 to 3 minutes decreases cardiac membrane excitability. The effect occurs in minutes but lasts only 30 to 60 minutes. It can be repeated after 5 to 10 minutes if the ECG does not change. Use with extreme caution in patients receiving digoxin.
•
Insulin
, 5 to 10 units IV, causes an intracellular shift of K
+
in 10 to 30 minutes. The effect lasts for several hours. Glucose, 100 g IV (2 amp D50), should also be administered to prevent hypoglycemia and the patient’s blood sugar should be checked in 1 to 2 hours.
•
NaHCO
3
1 ampule (i.e., 50 mmol HCO
3
-
in 50 mL) IV can also be used to cause an intracellular shift of K
+
, and the effect can last several hours. This treatment should probably be reserved for patients with severe hyperkalemia
and
metabolic acidosis. Patients with end-stage renal disease seldom respond and may not tolerate the Na
+
load.
•
β
2
-adrenergic agonists
can be used to cause an intracellular shift of K
+
.
•
Diuretics
(e.g., furosemide, 40 to 120 mg IV) enhance K
+
excretion provided renal function is adequate.
•
Cation exchange resins
(sodium polystyrene sulfonate; Kayexalate) enhance K
+
excretion from the GI tract, but single doses are only mildly effective. Though previously very common, the FDA now recommends that Kayexalate NOT be given in sorbitol solution due to the risk of intestinal necrosis. Kayexalate reconstituted in water may be given PO (15 to 30 g) or as a retention enema (50 g in 150 mL of tap water). It may be beneficial to coadminister an alternative laxative, such as lactulose or MiraLAX, when giving PO Kayexalate. Doses may be repeated q4-6h.
•
Dialysis
may be necessary for severe hyperkalemia when other measures are ineffective and for patients with renal failure.
•
Chronic treatment
is aimed at the underlying condition. Dietary K
+
should be restricted. Metabolic acidosis should be corrected. Drugs causing hyperkalemia should be avoided. Administration of exogenous mineralocorticoid may be effective for select patients.
Acid–Base Disorders
18
GENERAL PRINCIPLES
•
Changes in acid–base balance occur as a result of changes in [H
+
] and [HCO
3
-
].
