Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (118 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
2.35Mb size Format: txt, pdf, ePub
• Endovascular (eg, intra-arterial lysis, thrombectomy): w/o proven benefit over thrombolysis IV alone (
NEJM
2013;368:893, 904, 914); thus still experimental, ? consider for major vascular occlusions (distal ICA, prox MCA, esp basilar given high mortality or disability untreated) • BP: lower to <185/110 to consider lysis; if lyse keep <180/105 × 24 h (consider labetalol or nicardipine), o/w permissive HTN unless >220/120 or sx; if sx HoTN consider vasopressors • Initiate ASA w/in 24–48 h; avoid anticoagulation w/in 24 h of lysis; see below for long-term Rx • Cerebral edema → herniation: often occurs 1–5 d post large MCA or cerebellar strokes, ↑ risk in young. Temporize: elevate HOB >30°; mannitol ± 23% NaCl. Hemicraniectomy ↓ mortality (
Lancet Neurol
2007, 6:215). Neurosurgery consult in select MCA and all large cerebellar strokes.
Secondary stroke prevention (
NEJM
2012;366:1914)
•
Antiplatelet therapy
: different agents likely have similar efficacy
ASA
↓ death & repeat stroke; equal to warfarin in nonembolic stroke (
NEJM
2001;345:1444)
ASA
+
dipyrimadole
: sup to ASA (
Lancet
2006;367:1665), but bid dosing, HA → ↓ compliance
clopidogrel
: marginally sup to ASA, slightly ↑ ICH (
Lancet
1996:348:1329)
cilostazol: superior to ASA, less bleeding (
Lancet Neurol
2010;9:959)
clopidogrel + ASA not more effective than ASA alone and ↑ ICH (
Lancet
2004;364:331)
•
Anticoagulation (AC)
: not routinely indicated
Indications: cardiac/paradoxical emboli (except bacterial endocarditis); long segment extradural dissections; hypercoag state; bridge to CEA in sx carotid stenosis w/ongoing TIAs.
INR goal 2–3 for warfarin. Consider LMWH in Pts w/malignancy.
Hold off on AC
in large strokes for ~2–4 wk given risk of hemorrhagic conversion.
• Long-term SBP target 120–139 mmHg (
JAMA
2011;306:2137) • Statin: ↓ recurrent stroke w/ atorvastatin 80 mg, LDL goal <70 (
NEJM
2006;355:549) • Fluoxetine: ? improved motor recovery after 3 mo (
Lancet Neurol
2011;10:123) •
Carotid revascularization
CEA
(
if
surgical morbidity & mortality ≤6%) indicated for:
sx stenosis
70–99% (benefit ↑ for males, >75 y, ≤2 wk from stroke) → 65% ↓ RR of repeat stroke, slight benefit for 50–69% stenosis (
NEJM
1991;325:445;
Lancet
2004;363:915)
asx stenosis
70–90%, <79 y: 50% ↓ RR of repeat stroke (
Lancet
2004;363:1491 & 2010;376:1074)
stenting
: compared w/ CEA, periprocedural risk of stroke ↑ (esp. in elderly) & MI ↓ (although many asx), subsequent rates of stroke similar (
NEJM
2010;363:11;
Lancet
2010;376:1062)
Patent foramen ovale (PFO; in ~27% of population) (
NEJM
2005;353:2361)
• ↑ stroke risk: ≥4 mm separation, R→L shunting at rest, ↑ septal mobility, atrial septal aneurysm • If PFO & stroke/TIA: no benefit of warfarin over ASA (
Circ
2002;105:2625), but consider if at high risk for or has DVT/PE. No sig benefit shown for PFO closure so far, albeit studies small & w/ favorable trends (
NEJM
2012;366:991; 2013:1083 & 1092).
INTRACRANIAL HEMORRHAGE (ICH)
Classification by location
• Hemorrhagic strokes: intraparenchymal hemorrhage (IPH) & subarachnoid hemorrhage (SAH)
• Other ICH: epidural hematoma (EDH) & subdural hematoma (SDH)
Etiologies
• AVM, aneurysm, cerebral venous sinus thrombosis → IPH or SAH
• HTN (basal ganglia, cerebellum, brainstem), cerebral amyloid (lobar), tumor (esp. w/ melanoma, renal cell CA, chorio-CA, thyroid CA) → IPH
• Trauma → all locations (nb, IPH or SAH caused by trauma technically not a stroke)
Clinical manifestations (
Lancet Neurol
2005;4:662;
BMJ
2010;341:c5204)
• ↓ consciousness, N/V, HA, progressive focal neurologic deficits
•
SAH
: thunderclap HA, onset w/ exertion; nuchal pain/rigidity; LOC.
EDH
: initial lucid interval.
Workup
• STAT CT brain, angio (CT-A or conventional) if suspicious for vascular source
• LP to ✓ for xanthochromia if no evidence of ICH on CT and suspicious for SAH
• Coags (PT, PTT, INR)
Management
• Reverse coagulopathies w/ vit K & FFP, goal INR <1.4. Plt goal >100k; no clear evidence for plt transfusion if on ASA but may consider with expanding ICH; DDAVP if uremic.
• HOB elevation to 30–45°; strict BP control w/ arterial line, use nicardipine or labetalol gtt, goal SBP <160, for aneurysmal SAH <140, unless risk for hypoperfusion b/c of crit carotid stenosis
• SAH: surgical clipping vs. endovascular coiling (depending on location, comorbidities) of aneurysm/AVM; nimodipine to ↓ risk of vasospasm (monitor w/ TCDs), seizure Ppx
• Surgical evacuation: any EDH; SDH if >1 cm or rapid expansion; IPH: consider in younger Pts w/ ICH, data controversial, potential benefit in superficial IPH (
Lancet
2005, 365:387)
• Venous sinus thrombosis: start anticoagulation, manage ↑ ICP and seizures as needed
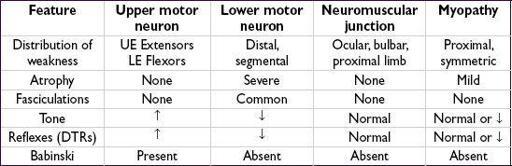
WEAKNESS & NEUROMUSCULAR DYSFUNCTION
PERIPHERAL NEUROPATHIES
Etiologies
•
Mononeuropathy
(one nerve): entrapment, compression, trauma, DM, Lyme.
Commonly seen: median n. (carpal tunnel syndrome); ulnar n. (at elbow or wrist); common peroneal n. (at knee with habitual leg crossing); lateral femoral cutaneous n. (at inguinal ligament).
•
Mononeuropathy multiplex
(axonal loss of multiple, separate, noncontiguous nerves):
vasculitides, sarcoid, DM, Lyme, Sjögren, hereditary neuropathy with pressure palsies
•
Small fiber neuropathy
: (unmyelinated or thinly myelinated nerves): idiopathic, DM, CTD, alcohol, sarcoid, thyroid dysfxn, B
12
defic, paraproteinemia, paraneo, celiac, hered.
•
Polyneuropathy
(multiple symmetric nerves, generally length dependent)
Demyelinating
acute: acute inflammatory demyelinating polyneuropathy (AIDP) = Guillain-Barré
subacute: meds (paclitaxel), paraneoplastic
chronic: idiopathic, DM, CIDP, hypothyroidism, toxins, paraproteinemia, hereditary
Axonal
acute: acute motor axonal neuropathy (AMAN), porphyria, vasculitis, uremia
subacute: DM, meds (cisplatin, paclitaxel, vincristine, INH, ddI), EtOH, sepsis, paraneo.
chronic: DM, uremia, lead, arsenic, HIV, paraproteinemia, B
12
defic
Clinical manifestations
• Weakness, fasciculations, numbness, dysesthesias (burning/tingling), allodynia • ± Autonomic dysfxn (orthostasis, bowel/bladder retention/incontinence, impotence) • Depressed or absent DTRs (may be normal in small fiber neuropathy)
Diagnostic studies
• Distal symmetric polyneuropathy: start w/ Hb
A1C
or glc tolerance test, B
12
, SPEP + SIEP
• EMG & NCS (often no change in first 10–14 d or in small fiber neuropathy) • Electrolytes, BUN/Cr, CBC, TSH, LFTs, ANA, anti-Ro, anti-La, ESR, HIV, Cu, Lyme titers, genetic testing and heavy metal screening as indicated by clinical history and exam • Autonomic testing/skin bx (small fiber), nerve bx (mononeuropathy multiplex) • MRI if possible radiculopathy or plexopathy (after EMG)
Treatment of neuropathic pain
• Pharmacologic: pregabalin, gabapentin, TCAs (nortriptyline, amitriptyline), SSRIs (duloxetine, venlafaxine), tramadol, topical analgesics (lidocaine, capsaicin), opiates • Nonpharmacologic: transcutaneous electrical nerve stimulation (TENS)
GUILLAIN-BARRÉ SYNDROME (GBS)
Definition & epidemiology
• Acute inflammatory demyelinating polyneuropathy (AIDP)
• Incidence 1–2 per 100,000; most common acute/subacute paralysis
• Precipitants in 60%: viral illness (CMV, EBV, HIV), URI (
Mycoplasma
), gastroenteritis (
Campylobacter
), Lyme, immunizations (no proven risk w/ current), surgery
Clinical manifestations
• Distal sensory dysesthesias and numbness often first symptoms, back pain also common • Ascending symmetric paralysis over hours to days; plateau in 1–3 wk • Hypoactive then absent reflexes
• Resp failure requiring mech vent occurs in 30%; autonomic instability & arrhythmias in 50%
• Fisher variant: ophthalmoplegia, ataxia, areflexia; associated with anti-GQ1b antibodies
Diagnostic studies (results may be normal in first several days)
• LP: albuminocytologic dissociation = ↑ protein w/o pleocytosis (<10 WBCs) seen in up to 50% of Pts in 1st wk, 75% by 3rd wk of symptoms • EMG & NCS: ↓ nerve conduction velocity, conduction block, prolonged F wave latency • FVC & NIF: to assess for risk of respiratory failure (cannot rely on P
a
O
2
or S
a
O
2
)
Treatment
• Plasma exchange (
Coch Data Syst Rev
2002;2:CD001798) or IVIg of equal efficacy and no additional benefit with both (
Neuro
2012;78:1009), steroids not beneficial • Supportive care with monitoring in ICU setting if rapid progression or resp. failure • Watch for autonomic dysfunction: labile BP, dysrhythmias (telemetry) • Most recover near baseline; axonal variant (~5%) with incomplete recovery; 3–5% mortality
MYASTHENIA GRAVIS
Definition & epidemiology
• Autoimmune disorder with Ab directed against acetylcholine receptor (AChR) in NMJ
• Prevalence: 1 in 7500; affects all ages, peak incidence 20s–30s (women), 60s–70s (men)
Clinical manifestations
• Fluctuating weakness w/
fatigability
(worse w/ repetitive use, relieved by rest)
• Cranial muscles involved early → ocular (ptosis, diplopia) in 50%; bulbar (difficulty
chewing, dysarthria, dysphagia) in 15%. Often later progresses to generalized weakness.
Other books
Self-Defense by Jonathan Kellerman
Cottonwood by Scott Phillips
The Holders by Scott, Julianna
Flash Point (Kilgore Fire Book 2) by Lani Lynn Vale
Children of the Uprising by Trevor Shane
Rescuing the Captive: The Ingenairii Series by Jeffrey Quyle
A Night Like This by Julia Quinn
Easy Silence by Beth Rinyu
The Instructions by Adam Levin