Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (49 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

• Clinical: asx vs. hepatic decompensation (eg, ascites, PSE), PVT w/ tumor thrombus • Dx: screen cirrhotics q6mo w/ U/S ± AFP, though many centers choose dual phase CT/MRI (if arterial enhancing & venous phase or delayed washout, no bx req for dx) • Rx:
radiofrequency ablation
(RFA) for HCCs <3 cm in size; consider
resection
if single lesion <2 cm and Child-Pugh A w/o portal HTN;
transarterial chemoembolization
(TACE) preferred for large cancers (not curative) or if not amenable to RFA (near IVC/lung); consider
liver transplant
if up to 3 HCCs ≤3 cm or 1 HCC ≤5 cm • Complications of Rx in 2–11%, procedure mortality ~0.5%. RFA → PVT, colon perforation, abscess, skin burn, PTX, subcapsular hematoma, AKI, diaphragm injury. TACE → postembolization syndrome (PES) = nausea, RUQ pain, ileus, fever, ↑ ALT/AST; self-limited, resolves w/in 1 wk. Other: hepatic ischemia, abscess (2%), biliary tree injury, cholecystitis, gastroduodenal ulceration (~5%), kidney injury (2%).
Other complications
•
Coagulopathy
(
NEJM
2011;365:147): complex balance of pro- & anti-hemostatic drivers ↑ bleeding: ↓ plts (sequestration & ↓ Tpo) & ↓ clotting factors, renal dysfxn ↑ clotting: ↑ vWF & factor VIII, ↓ protein C, S, ATIII •
Hepatopulmonary syndrome
(HPS) (
NEJM
2008;358:2378)
Definition/etiology: abnl pulm gas exchange (A-a gradient ≥15 or P
a
O
2
<80) + intrapulm vascular shunting w/o intrinsic pulm disease; ? due to ↑ pulmonary NO
S/S: platypnea-orthodeoxia, clubbing, cyanosis
Dx w/ contrast echo showing pulm A-V shunt (opac. in LA 3–6 cycles after RA)
Rx: O
2
; potential embolization if large vessel on CT, ? TIPS, liver tx only definitive Rx
•
Portopulmonary hypertension
(POPH) (
J Clin Gastr
2011;45:703): ↑ PAP (MPAP >25 mmHg), PVR >240 dyns/cm
5
and PAOP <15 mmHg. Due to pulm vasoconstriction from ↑ endothelin in ESLD. If PASP ≥40 mmHg by TTE → RHC.
•
Cirrhotic cardiomyopathy
: ↓ inotropic & chronotropic response, ↓ systolic and diastolic fxn, prolonged QT, hyperkinetic circulation; ↑ troponin, BNP (
JACC
2010;56:539) •
Infxn
: Kupffer cell (hepatic mΦ) dysfxn, ↓ opsonic activity; vaccinate for HAV & HBV, influenza yearly, pneumococcal vaccine, avoid PPIs? (
Alim Pharm Ther
2012;36:866) • Endocrine: diabetes (15–30%) due to altered glc & insulin metabolism; ↑ frequency of adrenal insufficiency in ESLD (
Hep
2012;55:1282)
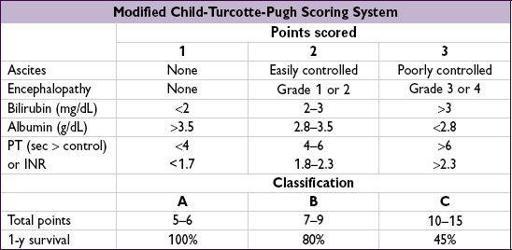
Prognosis
•
MELD
(Model for End-Stage Liver Disease): used to stratify Pts on liver tx list & to predict 3-mo survival in Pts w/ cirrhosis and some acute forms of liver disease. Based on Cr, INR, & total bili. Calculator:
www.mayoclinic.org/meld/mayomodel6.html
(
Gastro
2011;14:1952). If MELD <21 additional predictors of mortality include Na <130 (
NEJM
2008;359:1018;
Clin Gastro Hep
2009;7:1236), refractory ascites, ↑ HVPG and low QoL.
Liver transplantation
• Undertake evaluation when MELD ≥15
• Indic: recurrent/severe enceph, refractory ascites, SBP, recurrent variceal bleeding, HRS, HPS, HCC (if no single lesion is >5 cm
or
≤3 lesions with largest ≤3 cm), acute liver failure • Contraindic: inadequate social support, active substance abuse (EtOH w/in 6 mo), sepsis, significant comorbidity (eg, PoPH w/ MPAP ≥45 mmHg refractory to Rx), extrahepatic cancer, persistent noncompliance • Survival: 1-y up to 90%, 5-y up to 80%, though lower with HCV; autoimmune liver disease, such as AIH/PBC/PSC may recur in 10–30% of allografts
OTHER ETIOLOGIES OF CIRRHOSIS
Hemochromatosis
(
Hep
2011;54:328;
BMJ
2011;342:218)
•
Recessive disorder of iron sensing or transport leading to tissue iron deposition
•
HFE
mutations (85% of cases), typically C282Y homozygotes (~0.5% of N. European Caucasians), rarely C282Y/H63D compound heterozygotes; C282Y homozygotes: 28% of develop sx (88% lab abnl), and 1% of
develop sx (88% lab abnl), and 1% of develop sx (due to menses ↓ Fe load → later presentation). C282Y/H63D: only 1.5% manifest dis.
develop sx (due to menses ↓ Fe load → later presentation). C282Y/H63D: only 1.5% manifest dis.
• Non-HFE mutations: hemojuvelin, hepcidin, transferrin receptor 2, & ferroportin • 2° Fe overload: thalassemia, PRBC transfusion, MDS, EtOH, NASH (
NEJM
2012;366:348) • Sx: fatigue & arthralgias. In
advanced disease
(rare): bronze skin (melanin + iron), hypogonadism (esp. in juvenile onset), DM, arthropathy (MCP), CHF, infxns (
Vibrio
,
Listeria
,
Yersinia
), cirrhosis (↑ risk if EtOH/fatty liver disease; 15% risk of HCC). Disease also a/w ALS (H63D homozygotes) & porphyria.
• Dx: fasting iron sat >45% (iron/TIBC × 100%); ↑ ferritin (acute phase reactant, so poor Sp; often nl in young Pts). If ↑ iron sat. → ✓ HFE to confirm dx, imaging by MRI (black liver) If HFE&
ferritin >1000 ng/mL or ↑ LFTs → liver bx for quant Fe index & to stage fibrosis • Treatment: phlebotomy (250 mL = 1 unit, ~250 mg of Fe) qwk until Fe sat <50% & ferritin 50–100 μg/L, then q3–4mo; avoid vit C, PPI (↓ intestinal iron transport); deferoxamine or deferasirox if phleb. contraindic.; genetic counseling
Wilson’s disease
(
J Hep
2012;56:671)
• Recessive disorder of copper transport (mutation in
ATP7B
) → copper overload; primarily affects liver, but also other tissues (brain, eye) • Epidemiology: 1 in 40,000, majority present b/t 5 & 35 y/o, only 3% of Pts present >40 y/o • Extrahepatic s/s: neuro ψ disease, parkinsonism & movement disorder (hepatolenticular disease), Kayser-Fleischer rings (in 99% w/ neuro ψ but in <50% w/ hepatic disease), Coombs
hemolytic anemia, renal disease • Dx: ↑ 24-h urine Cu, ↓ serum ceruloplasmin (Se 90%), rarely penicillamine challenge w/ ↑ urine Cu excretion, liver bx w/ hepatic Cu content. In
acute liver failure
, AΦ/bili <4 + AST/ALT >2.2 better Se & Sp than urine Cu or ceruloplasmin (
Hepatology
2008;4:1167).
•
Treatment: chelation
w/ penicillamine + pyridoxine; 2nd line trientine (↓ toxicity w/ similar efficacy). Zinc: ↓ intestinal Cu transport and can help delay disease; best used if asx or in conjunction w/ chelation (must give 4–5 h apart from chelators).
ɑ
1
-antitrypsin deficiency (ɑ
1
-AT) (
NEJM
2009;360:2749;
Clin Gas Hep
2012;10:575)
• Abnl ɑ
1
-AT → polymerization in liver (cirrhosis) & uninhibited protease activity in lung (emphysema). Affects 1/3000 of European ancestry. Varied presentations: neonatal hepatitis in infants; cholestatic jaundice in kids; ↑ AST/ALT or cirrhosis in kids/adults.
• Extrahepatic disease: emphysema, necrotizing panniculitis, ANCA vasculitis (Wegener) • Dx: serum ɑ
1
-AT level (acute phase reactant), level <50% of nl typically diagnostic;
gold standard = phenotyping of protease inhibitor (Pi); Z is high-risk allele (ZZ = liver dis); S is “slow” allele (SZ = liver or lung dz); M is nl (MZ ? ↑ risk of dis); null/null → no ɑ
1
-AT protein, ∴ only emphysema and not liver dis (no polymerization)